| Active Ingredient | VISMODEGIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERIVEDGE | (NDA) 203388 | GENENTECH | CAPSULE;ORAL | 150MG | 150MG (RS) | January 30, 2012 | January 30, 2017 | - | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
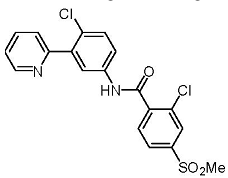 |
| Chemical Name | 2-Chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide |
| CAS No | 879085-55-9 |
| Molecular Formula | C19 H14 Cl2N2O3S |
| Molecular Weight | 421.30 g/mol |
| Appearance | a crystalline free base, appearing as a white to tan solid |
| Solubility | It is practically insoluble in water, soluble in acetone, sparingly soluble in acetonitrile and slightly soluble in ethanol. |
| Water Solubility | 0.1 μg/mL and is pH dependent |
| Polymorphism | Vismodegib primarily exists as polymorph B, the most thermodynamically stable polymorph observed to date. Formation of the desired polymorphic form during manufacture of vismodegib is assured by seeding with crystals of Polymorph B and by drying to ensure low solvate and thus low residual solvent levels. In addition, the crystal form is confirmed by an X-ray powder diffraction analysis on the final active substance. Form B of vismodegib is the only form that has been used in clinical development. Sufficient evidence was provided to demonstrate that Form B is obtained by the employed manufacturing process of the active substance. The crystallization solvents and conditions to generate Forms C and E are drastically different from the conditions used in vismodegib processing. Form B is monotropically more stable than Forms A and C, and is enantiotropically more stable than Form E at lower temperature. |
| pKa (Strongest Acidic) | 3.8 (pyridinium cation) |
| pKa (Strongest Basic) | - |
| Log P | 2.7 |
| Identification | FTIR, HPLC and XRPD |
| Degradation | Vismodegib is stable under light, heat, and humidity, with some degradation observed under acidic and basic stress conditions. Degradation was also observed when vismodegib was exposed to oxidative reagent (H2O2) for a longer period. In the photostability study the appearance of the substance was changed from white to light orange, no changes were seen for the other parameters. |
| Hygroscopic | non hygroscopic |
| Photostability study | Photo sensitive |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | During development, the critical quality attributes (CQAs) of the active substance were established based on the potential to impact the performance and manufacturability of the finished product and these include impurities (organic and inorganic impurities, residual solvents, water, and metals), polymorph form, and particle size. The synthesis of vismodegib involves three steps which comprise formation and/or breaking of chemical bonds. The particle size of the dried vismodegib is reduced by milling to obtain the desired particle size distribution. |
| Parameters | Details |
|---|---|
| Indications and Usage | ERIVEDGE capsule is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation. |
| Dosage and Administration | The recommended dose of ERIVEDGE is 150 mg taken orally once daily until disease progression or until unacceptable toxicity. ERIVEDGE may be taken with or without food. Swallow capsules whole. Do not open or crush capsules. If a dose of ERIVEDGE is missed, do not make up that dose; resume dosing with the next scheduled dose. |
| Mechanism of action | Vismodegib is an inhibitor of the Hedgehog pathway. Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction. |
| Absorption | The single dose absolute bioavailability of vismodegib is 31.8%. Absorption is saturable as evidenced by the lack of dose proportional increase in exposure after a single dose of 270 mg or 540 mg vismodegib. |
| Food Effect | ERIVEDGE capsule may be taken without regard to meals because the systemic exposure of vismodegib at steady state is not affected by food. |
| Distribution | The volume of distribution of vismodegib ranges from 16.4 to 26.6 L. Vismodegib plasma protein binding in patients is greater than 99%. Vismodegib binds to both human serum albumin and alpha-1-acid glycoprotein (AAG) and binding to AAG is saturable. In a pharmacokinetic study, male patients (n=3) had an average concentration of vismodegib in semen on day 8 that was 6.5% of the average steady state concentration (Css) observed in plasma. |
| Metabolism | Greater than 98% of the total circulating drug-related components are the parent drug. Metabolic pathways of vismodegib in humans include oxidation, glucuronidation, and pyridine ring cleavage. The two most abundant oxidative metabolites recovered in feces are produced in vitro by recombinant CYP2C9 and CYP3A4/5. |
| Elimination | Vismodegib and its metabolites are eliminated primarily by the hepatic route with 82% of the administered dose recovered in the feces and 4.4% recovered in urine. The estimated elimination half-life (t 1/2 ) of vismodegib is 4 days after continuous once-daily dosing and 12 days after a single dose. |
| Peak plasma time (Tmax) | 1 hr to 7 days (Vismodegib plasma concentrations rose relatively fast, but the initial absorption phase was followed by a broad peak.) |
| Half life | 4 days |
| Bioavailability | 31.80% |
| Age, gender | The results of a population pharmacokinetic analysis suggested no clinically relevant effect of weight (range: 41-140 kg), age (range: 26-89 years), and sex on the systemic exposure of vismodegib. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 150MG |
| Excipients used | microcrystalline cellulose (87.3MG), lactose monohydrate (71.5MG), sodium lauryl sulfate (7MG), povidone (10.5MG), sodium starch glycolate (17MG), talc (3.5MG), and magnesium stearate (non-bovine) (1.7MG) |
| Composition of coating material | - |
| Composition of caspule shell | The capsule shell contains gelatin, titanium dioxide, red iron oxide, and black iron oxide. The black printing ink contains shellac and black iron oxide. |
| Pharmaceutical Development | the development of the wet granulated formulation focus was set on compendial capsule dosage form parameters (dissolution, content uniformity (UoC) and stability), active substance physical characteristics and the excipients sodium lauryl sulfate and magnesium stearate, since the drug substance was considered to be a BCS Class 2 compound. Factors not considered to be critical or having a significant effect were e.g. water content, stability of drug substance (stable), polymorphic form (since it was established that the B form was achieved in the manufacturing and it is thermodynamically stable) and level of disintegrant since the microcrystalline cellulose filler also act as disintegrant. |
| Manufacture of the product | The hard capsules are manufactured by a standard process comprising wet granulation in high-shear granulator where the active substance, microcrystalline cellulose, lactose monohydrate, sodium lauryl sulfate, and part of sodium starch glycollate is granulated with the granulation solution consisting of povidone and purified water. The granules are dried in a fluid-bed, milled and mixed with sodium starch glycollate and talc followed by mixing with magnesium stearate and filling. |
| Tablet / Capsule Image |
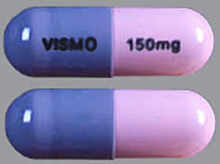
|
| Appearance | The capsule has a pink opaque body and a grey opaque cap, with “150 mg” printed on the capsule body and “VISMO” printed on the capsule cap in black ink. |
| Imprint code / Engraving / Debossment | “150 mg” printed on the capsule body and “VISMO” printed on the capsule cap in black ink |
| Score | no score |
| Color | pink opaque body and a grey opaque cap |
| Shape | Capsule |
| Dimension | 19mm |
| Mfg by | Patheon, Inc. (US), Roche Pharma (EU) |
| Mfg for | - |
| Marketed by | - |
| Distributed by | Genentech Inc. (US), Roche Pharma (EU) |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N203388 | 1 | 7888364 | November 11, 2028 | Y | Y | - | - | Download |
| N203388 | 1 | 9278961 | December 15, 2028 | - | - | U - 1825 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.01 N HCl with 1.0% sodium lauryl sulfate (SLS) | 900 | 10, 15, 20, 30 and 45 | May 28, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | ERIVEDGE | Download |
| UK | ERIVEDGE | Download |
| US | ERIVEDGE | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
