| Active Ingredient | VENETOCLAX |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VENCLEXTA | (NDA) 208573 | ABBVIE INC | TABLET;ORAL | 10MG, 50MG, 100MG | 100MG | April 11, 2016 | April 11, 2021 | April 11, 2023 | 1 New molecular entity (NME) | P Priority review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
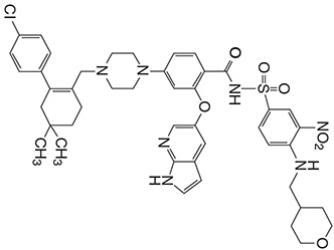 |
| Chemical Name | 4-(4-{[2-(4-chlorophenyl)-4,4dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4ylmethyl)amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide) |
| CAS No | 1257044-40-8 |
| Molecular Formula | C45H50ClN7O7S |
| Molecular Weight | 868.44 |
| Appearance | light yellow to dark yellow solid |
| Solubility | Venetoclax has very low aqueous solubility. It is very slightly soluble in 1% polysorbate 80 (w/v aq.) |
| Water Solubility | As a function of pH in aqueous buffer, aqueous solubility is <0.0042 µg/mL at pH 7.4 . |
| Polymorphism | Polymorphism has been observed for venetoclax. Multiple crystal forms have been discovered in solid form screening studies. The thermodynamically stable form is consistently manufactured and does not change upon storage. |
| pKa (Strongest Acidic) | 3.4 and 10.3 for the sulfonamide and piperazine groups respectively |
| pKa (Strongest Basic) | 7.96 |
| Log P | 5.5 |
| Identification | IR, HPLC |
| Degradation | Venetoclax is sensitive to oxidation and slightly sensitive to UV radiation, heat, heat and moisture and acid treatment. Although stress studies under basic conditions were not performed, early physicochemical properties studies on venetoclax demonstrated that the active substance is stable under alkaline conditions. |
| Hygroscopic | Non hygroscopic |
| Photostability study | Venetoclax exhibits sensitivity to light. |
| Melting Point | 138˚C |
| BCS Class | II or IV |
| Manufacture of API | Venetoclax is synthesized in four main stages, using four well defined starting materials with acceptable specifications. Each manufacturing stage is divided into unit operations and each unit operation may have one or more process steps to produce the crystalline form selected for manufacture. Several manufacturing sites are involved in the manufacture of the active substance. Where common intermediates are manufactured by different sites, identical synthetic route and materials (catalyst, reagents and solvents) are used. |
| Parameters | Details |
|---|---|
| Indications and Usage | VENCLEXTA is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. |
| Dosage and Administration |
Administer the VENCLEXTA dose according to a weekly ramp-up schedule over 5 weeks to the recommended daily dose of 400 mg as 1, 2, 3, 4 week dose are 20MG, 50MG, 100MG and 200MG respectively. Refer FDA Label for more information. |
| Mechanism of action | Venetoclax is a selective and orally bioavailable small-molecule inhibitor of BCL-2, an antiapoptotic protein. Overexpression of BCL-2 has been demonstrated in CLL cells where it mediates tumor cell survival and has been associated with resistance to chemotherapeutics. Venetoclax helps restore the process of apoptosis by binding directly to the BCL-2 protein, displacing pro-apoptotic proteins like BIM, triggering mitochondrial outer membrane permeabilization and the activation of caspases. In nonclinical studies, venetoclax has demonstrated cytotoxic activity in tumor cells that overexpress BCL-2. |
| Absorption | Following multiple oral administrations under fed conditions, maximum plasma concentration of venetoclax was reached 5-8 hours after dose. Venetoclax steady state AUC increased proportionally over the dose range of 150-800 mg. Under low-fat meal conditions, venetoclax mean (± standard deviation) steady state Cmax was 2.1 ± 1.1 μg/mL and AUC0-24 was 32.8 ± 16.9 μg•h/mL at the 400 mg once daily dose. |
| Food Effect | Administration with a low-fat meal increased venetoclax exposure by approximately 3.4-fold and administration with a high-fat meal increased venetoclax exposure by 5.1- to 5.3-fold compared to fasting conditions. Venetoclax should be administered with a meal. |
| Distribution | Venetoclax is highly bound to human plasma protein with unbound fraction in plasma <0.01 across a concentration range of 1-30 µM (0.87-26 µg/mL). The mean blood-to-plasma ratio was 0.57. The population estimate for apparent volume of distribution (Vdss/F) of venetoclax ranged from 256-321 L in patients. |
| Metabolism |
The population estimate for the terminal elimination half-life of venetoclax was approximately 26 hours. The pharmacokinetics of venetoclax does not change over time. In vitro studies demonstrated that venetoclax is predominantly metabolized by CYP3A4/5. M27 was identified as a major metabolite in plasma with an inhibitory activity against BCL-2 that is at least 58-fold lower than venetoclax in vitro. |
| Elimination | After single oral administration of 200 mg radiolabeled [14C]-venetoclax dose to healthy subjects, >99.9% of the dose was recovered in feces and <0.1% of the dose was excreted in urine within 9 days, indicating that hepatic elimination is responsible for the clearance of venetoclax from the systemic circulation. Unchanged venetoclax accounted for 20.8% of the administered radioactive dose excreted in feces. |
| Peak plasma time (Tmax) | 5-8 hours |
| Half life | 26 hours |
| Bioavailability | - |
| Age, gender | Based on population pharmacokinetic analyses, age, race, sex, and weight do not have a clinically meaningful effect on venetoclax clearance. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 10MG | 50MG | 100MG | |
| Excipients used | Copovidone (k28, 66.6 mg), colloidal silicon dioxide (1.9 mg), polysorbate 80 (5.8 mg), sodium stearyl fumarate (0.5 mg), and calcium phosphate dibasic (21.2 mg) |
Copovidone (K28, 333.4 mg), colloidal silicon dioxide (9.6 mg), polysorbate 80 (29.2 mg), sodium stearyl fumarate (2.6 mg), and calcium phosphate dibasic (106.2 mg) |
Copovidone (K28, 666.8 mg), colloidal silicon dioxide (18.9mg), polysorbate 80 (53.3 mg), sodium stearyl fumarate (5.3 mg), and calcium phosphate dibasic (212.3 mg) |
|
| Composition of coating material | iron oxide yellow, polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide (Opadry II85F32450, 4.2 mg) | iron oxide yellow, iron oxide red, iron oxide black, polyvinyl alcohol, talc, polyethylene glycol and titanium dioxide (Opadry II85F97497 beige, 21.2 mg) | iron oxide yellow, polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide (Opadry II85F32450, 42.5 mg) | |
| Composition of caspule shell | - | |||
| Pharmaceutical Development |
The three strengths are dose proportional. Venetoclax active substance is an ionisable compound with two pKa values of physiological importance (3.4, acidic sulfonamide and 10.3, basic piperazine). The active substance has very poor aqueous solubility. Because of the very low solubility characteristics, a solid dispersion approach in copovidone (manufacturing an intermediate), is employed to increase the apparent aqueous solubility and bioavailability of venetoclax. The good solubility and compatibility of venetoclax in copovidone was confirmed by DSC studies. |
|||
| Manufacture of the product | The manufacturing process for venetoclax, includes manufacture of the intermediate, milling, blending,tableting, coating and packaging. | |||
| Tablet / Capsule Image |

|

|
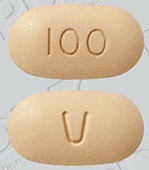
|
|
| Appearance | film-coated tablets are round, biconvex shaped, pale yellow debossed with “V” on one side and “10” on the other side | film-coated tablets are oblong, biconvex shaped, beige debossed with “V” on one side and “50” on the other side | film-coated tablets are oblong, biconvex shaped, pale yellow debossed with “V” on one side and “100” on the other side. | |
| Imprint code / Engraving / Debossment | debossed with “V” on one side and “10” | debossed with “V” on one side and “50” | debossed with “V” on one side and “100" | |
| Score | no score | no score | no score | |
| Color | Pale yellow | Beige | Pale yellow | |
| Shape | ROUND | OBLONG | OBLONG | |
| Dimension | 6mm | 14mm | 17mm | |
| Mfg by | AbbVie Inc.(US) | |||
| Mfg for | - | |||
| Marketed by | AbbVie Inc. & Genentech (US) | |||
| Distributed by | - | |||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N208573 | 1 | 8546399 | June 27, 2031 | Y | Y | - | - | Download |
| N208573 | 1 | 9174982 | May 26, 2030 | - | - | U - 1835 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| III (Reciprocating Cylinder) [Bottom Screen: 200 mesh stainless steel] | 20 dpm | Phosphate Buffer, pH 6.8 with 0.4% sodium dodecyl sulfate (SDS) [3 small drops of antifoaming agent may be used] | 250 | 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 3.5 and 4 hours | July 28, 2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | VENCLEXTA | Download |
| UK | VENCLEXTA | Download |
| US | VENCLEXTA | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
