| Active Ingredient | VALBENAZINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INGREZZA | NDA#209241 | NEUROCRINE BIOSCIENCES INC | CAPSULE;ORAL | 40MG | 40MG | April 11, 2017 | April 11, 2022 | - | 1 New molecular entity (NME) | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
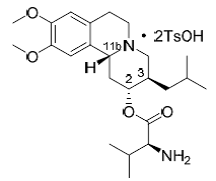 |
| Chemical Name | L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10- dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester, 4-methylbenzenesulfonate (1:2) |
| CAS No | 1025504-45-3 |
| Molecular Formula | C38H54N2O10S2 (ditosylate salt), C24H38N2O4 (Free Base) |
| Molecular Weight | 762.97 g/mol (ditosylate salt), 418.57 (Free Base) |
| Appearance | - |
| Solubility | Valbenazine tosylate is slightly soluble in water. |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | 8.41 (Predicted) |
| pKa (Strongest Basic) | - |
| Log P | 3.63 (Predicted) |
| Identification | - |
| Degradation | - |
| Hygroscopic | Slightly Hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | INGREZZA is indicated for the treatment of adults with tardive dyskinesia |
| Dosage and Administration |
The initial dose for INGREZZA is 40 mg once daily. After one week, increase the dose to the recommended dose of 80 mg once daily. Continuation of 40 mg once daily may be considered for some patients. Administer INGREZZA orally with or without food. Refer FDA label for more details. |
| Mechanism of action |
valbenazine is a vesicular monoamine transporter 2 (VMAT2) inhibitor. The mechanism of action of valbenazine in the treatment of tardive dyskinesia is unknown, but is thought to be mediated through the reversible inhibition of vesicular monoamine transporter 2 (VMAT2), a transporter that regulates monoamine uptake from the cytoplasm to the synaptic vesicle for storage and release. |
| Absorption |
Valbenazine and its active metabolite ([+]-α-HTBZ) demonstrate approximate proportional increases for the area under the plasma concentration versus time curve (AUC) and maximum plasma concentration (Cmax) after single oral doses from 40 mg to 300 mg (i.e., 50% to 375% of the recommended treatment dose). Following oral administration, the time to reach maximum valbenazine plasma concentration (tmax) ranges from 0.5 to 1.0 hours. Valbenazine reaches steady state plasma concentrations within 1 week. The absolute oral bioavailability of valbenazine is approximately 49%. [+]-α-HTBZ gradually forms and reaches Cmax 4 to 8 hours after administration of INGREZZA. |
| Food Effect | Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 47% and AUC by approximately 13%. [+]-α-HTBZ Cmax and AUC are unaffected. |
| Distribution |
The plasma protein binding of valbenazine and [+]-α-HTBZ are greater than 99% and approximately 64%, respectively. The mean steady state volume of distribution of valbenazine is 92 L. Nonclinical data in Long-Evans rats show that valbenazine can bind to melanin-containing structures of the eye such as the uveal tract. The relevance of this observation to clinical use of INGREZZA is unknown. |
| Metabolism |
Valbenazine is extensively metabolized after oral administration by hydrolysis of the valine ester to form the active metabolite ([+]-α-HTBZ) and by oxidative metabolism, primarily by CYP3A4/5, to form monooxidized valbenazine and other minor metabolites. [+]-α-HTBZ appears to be further metabolized in part by CYP2D6. The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 or CYP3A4/5, or induce CYP1A2, CYP2B6 or CYP3A4/5 at clinically relevant concentrations. The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit the transporters (BCRP, OAT1, OAT3, OCT2, OATP1B1, or OATP1B3) at clinically relevant concentrations. |
| Elimination | Following the administration of a single 50-mg oral dose of radiolabeled C-valbenazine (i.e., ~63% of the recommended treatment dose), approximately 60% and 30% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 2% was excreted as unchanged valbenazine or [+]-α-HTBZ in either urine or feces. |
| Peak plasma time (Tmax) | 4 to 8 hours |
| Half life | 15 to 22 hours |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 40MG |
| Excipients used | mannitol, partially pregelatinized starch, fumed silica, and magnesium stearate |
| Composition of coating material | - |
| Composition of caspule shell | gelatin, candurin silver fine, FD&C Red#40, and FD&C Blue#1 |
| Pharmaceutical Development | Each capsule contains 73 mg of valbenazine tosylate, which is equivalent to 40 mg of valbenazine free base. |
| Manufacture of the product | - |
| Tablet / Capsule Image | |
| Appearance | white opaque body and purple cap capsule is printed with ‘VBZ’ and ‘40’ in black ink |
| Imprint code / Engraving / Debossment | body and purple cap capsule is printed with ‘VBZ’ and ‘40’ in black ink |
| Score | no score |
| Color | White |
| Shape | Capsule |
| Dimension | Size 1 |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by | Neurocrine Biosciences, Inc. |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N209241 | 1 | 8039627 | October 6, 2029 | DS | DP | - | - | Download |
| N209241 | 1 | 8357697 | November 8, 2027 | - | - | U-1995 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) with sinker | 50 | Tier 1: 0.1N HCl; Tier 2: 0.1N HCl containing pepsin (750,000 units per 1000 mL) | 900 | 5, 10, 15, 20 and 30 | November 2, 2017 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
