| Active Ingredient | TRICLABENDAZOLE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGATEN | 208711 | NOVARTIS PHARMS CORP | TABLET;ORAL | 250MG | 250MG | February 13, 2019 | February 13, 2024 | February 13, 2026 | Type 1 - New Molecular Entity | PRIORITY; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
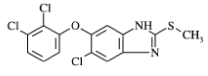 |
| Chemical Name | 6-chloro-5-(2, 3-dichlorophenoxy)-2-(methylthio)-1Hbenzimidazole (triclabendazole) |
| CAS No | 68786-66-3 |
| Molecular Formula | C14H9Cl3N2OS |
| Molecular Weight | 359.65 g/mol |
| Appearance | white or almost white, crystalline powder |
| Solubility | Soluble in tetrahydrofuran, cyclohexanone, acetone, iso- propanol, n-octanol,and methanol; slightly soluble in dichloro-methane, chloroform, toluene, xylene, ethyl acetate; insoluble in water, hexane. |
| Water Solubility | Insoluble in water |
| Polymorphism | - |
| pKa (Strongest Acidic) | 10.46 |
| pKa (Strongest Basic) | 4.54 |
| Log P | 5.5 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 175–176°C |
| BCS Class | II/IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | EGATEN™ tablet is an anthelmintic indicated for the treatment of fascioliasis in patients 6 years of age and older |
| Dosage and Administration |
• The recommended dose of EGATEN is 2 doses of 10 mg/kg given 12 hours apart in patients 6 years of age and older. • Take orally with food. • Swallow tablets whole or divide in half and take with water, or crush and administer with applesauce. • If the dosage cannot be adjusted exactly, round dose upwards. |
| Mechanism of action | The mechanism by which triclabendazole exhibits its effect against Fasciola species is not fully elucidated.Studies in vitro and/or in infected animals suggest that triclabendazole and its active metabolites (sulfoxide and sulfone) are absorbed by the tegument of the immature and mature worms, leading to a decrease of the resting membrane potential, inhibition of tubulin function as well as protein and enzyme synthesis. These metabolic disturbances are associated with inhibition of motility, disruption of the surface as well as ultrastructure that includes inhibition of spermatogenesis and vitelline cells. |
| Absorption |
After oral administration of a single dose of 10 mg/kg triclabendazole with a 560-kcal meal to patients with fascioliasis, mean peak plasma concentrations (Cmax) for triclabendazole, the sulfoxide and sulfone metabolites were 1.16, 38.6, and 2.29 µmol/L, respectively. The area under the curve (AUC) for triclabendazole, the sulfoxide and sulfone metabolites were 5.72, 386, and 30.5 µmol∙h/L, respectively. Absorption Following oral administration of a single dose of triclabendazole at 10 mg/kg with a 560-kcal meal to patients with fascioliasis, the median Tmax for the parent compound and the sulfoxide metabolite was 3 to 4 hours. |
| Food Effect | Cmax and AUC of triclabendazole and sulfoxide metabolite increased approximately 3-fold and 2-fold respectively when triclabendazole was administered as a single dose at 10 mg/kg with a meal containing a total of approximately 560 kcal (consisting of 2 cups of sweetened white coffee, a roll with cheese, and a roll with butter and jam). In addition, the sulfoxide metabolite Tmax increased from 2 hours in the fasted state to 4 hours in the fed state. |
| Distribution | The apparent volume of distribution (Vd) of the sulfoxide metabolite in fed patients is approximately 1 L/kg.Protein-binding of triclabendazole, sulfoxide metabolite and sulfone metabolite in human plasma was 96.7%,98.4% and 98.8% respectively. |
| Metabolism | Based on in vitro studies, triclabendazole is primarily metabolized by CYP1A2 (approximately 64%) into its active sulfoxide metabolite and to a lesser extent by CYP2C9, CYP2C19, CYP2D6, CYP3A, and FMO. This sulfoxide metabolite is further metabolized primarily by CYP2C9 to the active sulfone metabolite and to a lesser extent by CYP1A1, CYP1A2, CYP1B1, CYP2C19, CYP2D6 and CYP3A4, in vitro. |
| Elimination |
Elimination The plasma elimination half-life (t1/2) of triclabendazole, the sulfoxide and sulfone metabolites in human is approximately 8, 14, and 11 hours, respectively. Excretion No excretion data is available in humans. However, in animals, the drug is largely excreted via the biliary tract in the feces (90%), together with the sulfoxide and sulfone metabolite. Less than 10% of an oral dose is excreted in the urine. |
| Peak plasma time (Tmax) | 3 to 4 hours. Tmax increased from 2 hours in the fasted state to 4 hours in the fed state. |
| Half life | Triclabendazole, the sulfoxide and sulfone metabolites in human is approximately 8, 14, and 11 hours, respectively. |
| Bioavailability | - |
| Age, gender |
The pharmacokinetics of EGATEN were not studied in patients with renal or hepatic impairment. Pediatric Patients No dedicated pediatric pharmacokinetic studies were conducted. However, in one pharmacokinetic study of 20 patients, 7 children (ages 9 to 15 years) were dosed with triclabendazole 10 mg/kg single dose. AUC values of triclabendazole sulfoxide were 20% lower in these pediatric patients in the fed state than in the 13 patients above 15 years of age, but the difference was not statistically significant |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 250 MG |
| Excipients used | Colloidal silicon dioxide,lactose monohydrate, maize starch, magnesium stearate |
| Composition of coating material | Iron oxide red, methylhydroxyethylcellulose |
| Composition of caspule shell | NA |
| Pharmaceutical Development | Updated soon.. |
| Manufacture of the product | Updated soon.. |
| Tablet / Capsule Image | |
| Appearance | Pale red, speckled, capsule shaped, biconvex tablets, with imprint “EG EG” on one side and functionally scored on both sides |
| Imprint code / Engraving / Debossment | Imprint “EG EG” on one side |
| Score | Functionally scored |
| Color | Pale red |
| Shape | Speckled, capsule shaped, biconvex |
| Dimension | 19mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by |
Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.1M HCl with 1% Tween 20; 37 ± 0.5°C | 1000 | Q point at 30 min | As per SBOA |
| Market | EU | US |
|---|---|---|
| Strength | Packaging System | |
| 250 MG | - | Blister packs of 4 tablets (NDC 0078-0937-91). |
| Storage | Store in the original container. Store below 30°C (86°F). | |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| Based on the stability data, the proposed shelf life of 24 months is acceptable when stored below 30°C. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
