| Active Ingredient | TICAGRELOR |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRILINTA | (NDA) 022433 | ASTRAZENECA LP | TABLET;ORAL | 60MG, 90MG | 60MG, 90MG (RS) | July 20, 2011 | - | - | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
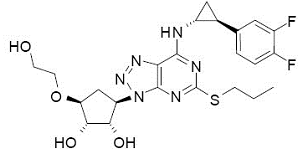 |
| Chemical Name | (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5(propylthio)-3H-[1,2,3]-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol |
| CAS No | 274693-27-5 |
| Molecular Formula | C23H28F2N6O4S |
| Molecular Weight | 522.57 |
| Appearance | a white or off-white to pale pink crystalline powder |
| Solubility | It's aqueous solubility of approximately 10 μg/mL at room temperature and does not exhibit pH dependent solubility and is defined as ‘low solubility’. |
| Water Solubility | 10 μg/mL |
| Polymorphism | Extensive polymorph screening has confirmed the existence of several crystal modifications of ticagrelor. There are 4 non-solvated polymorphs (Polymorph I, II, III and IV) and a number of solvated crystalline modifications that are clearly distinguishable by X-Ray Powder Diffraction. |
| pKa (Strongest Acidic) | 12.94 (Predicted) |
| pKa (Strongest Basic) | 2.93 (Predicted) |
| Log P | 2.31 (Predicted) |
| Identification | IR |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | IV |
| Manufacture of API | The commercial manufacturing process comprises 6 steps and is based on 3 starting materials for which acceptable specifications have been presented. Ticagrelor has 6 stereocenters resulting in 64 theoretical stereoisomers including the ticagrelor molecule itself. Control of the isomers is ensured with a four-step strategy: (1) stereoselective chemistry early in the synthetic pathway (2) stereochemical control of impurities in the specification of the proposed starting materials (3) thorough understanding of the potential formation of stereochemical impurities during the manufacturing process (4) thorough knowledge of the stereochemical impurities potentially present. The polymorphic form is relevant in view of the low solubility of the active substance and the solid oral dosage form in which it is presented. Consistent manufacture of the same polymorphic form is therefore essential; this has been sufficiently confirmed by batch analyses which confirm also that epimerisation does not take place during synthesis or routine storage. |
| Parameters | Details |
|---|---|
| Indications and Usage | BRILINTA is indicated to reduce the rate of cardiovascular death,myocardial infarction, and stroke in patients with acute coronary syndrome (ACS) or a history of myocardial infarction (MI). For at least the first 12 months following ACS, it is superior to clopidogrel. BRILINTA also reduces the rate of stent thrombosis in patients who have been stented for treatment of ACS. |
| Dosage and Administration |
In the management of ACS, initiate BRILINTA treatment with a 180 mg loading dose. Administer 90 mg twice daily during the first year after an ACS event. After one year administer 60 mg twice daily. Do not administer BRILINTA with another oral P2Y12 platelet inhibitor. Use BRILINTA with a daily maintenance dose of aspirin of 75-100 mg. A patient who misses a dose of BRILINTA should take one tablet (their next dose) at its scheduled time. For patients who are unable to swallow tablets whole, BRILINTA tablets can be crushed, mixed with water and drunk. The mixture can also be administered via a nasogastric tube (CH8 or greater). |
| Mechanism of action | Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent. |
| Absorption |
Ticagrelor demonstrates dose proportional pharmacokinetics, which are similar in patients and healthy volunteers. BRILINTA can be taken with or without food. Absorption of ticagrelor occurs with a median tmax of 1.5 h (range 1.0– 4.0). The formation of the major circulating metabolite AR-C124910XX (active) from ticagrelor occurs with a median tmax of 2.5 h (range 1.5-5.0). The mean absolute bioavailability of ticagrelor is about 36% (range 30%-42%). BRILINTA as crushed tablets mixed in water, given orally or administered through a nasogastric tube into the stomach, is bioequivalent to whole tablets (AUC and Cmax within 80-125% for ticagrelor and AR-C124910XX) with a median tmax of 1.0 hour (range 1.0 – 4.0) for ticagrelor and 2.0 hours (range 1.0 –8.0) for AR-C124910XX. |
| Food Effect | Ingestion of a high-fat meal had no effect on ticagrelor Cmax, but resulted in a 21% increase in AUC. The Cmax of its major metabolite was decreased by 22% with no change in AUC. |
| Distribution | The steady state volume of distribution of ticagrelor is 88 L.Ticagrelor and the active metabolite are extensively bound to human plasma proteins (>99%). |
| Metabolism | CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation ofits major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30-40%of the exposure of ticagrelor. |
| Elimination | The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion. The mean t1/2 is approximately 7 hours for ticagrelor and 9 hours for the active metabolite. |
| Peak plasma time (Tmax) | 1.5 h (range 1.0– 4.0) |
| Half life | 7 hours for ticagrelor and 9 hours for the active metabolite |
| Bioavailability | 36% (range 30%-42%) |
| Age, gender | The effects of age, gender and ethnicity are modest and do not require dose adjustment. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 28110 | A | II | February 20, 2014 | KAIYUAN HENGTAI PHARMA CO LTD (TICAGRELOR INTERMEDIATE) |
| 28111 | A | II | February 20, 2014 | KAIYUAN HENGTAI PHARMA CO LTD (TICAGRELOR INTERMEDIATE) |
| 28112 | A | II | February 20, 2014 | KAIYUAN HENGTAI PHARMA CO LTD (TICAGRELOR INTERMEDIATE) |
| 28446 | A | II | August 28, 2014 | DR REDDYS LABORATORIES LTD |
| 28456 | A | II | January 7, 2014 | KAIYUAN HENGTAI PHARMA CO LTD |
| 28674 | A | II | September 24, 2014 | ZAKLADY FARMACEUTYCZNE POLPHARMA SA |
| 28787 | A | II | January 30, 2015 | HEC PHARM CO LTD |
| 28812 | A | II | October 12, 2014 | MYLAN LABORATORIES LTD |
| 28879 | A | II | July 1, 2015 | HONOUR LAB LTD |
| 28880 | A | II | January 21, 2015 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 28899 | A | II | September 1, 2015 | ALEMBIC PHARMACEUTICALS LTD |
| 28915 | A | II | June 2, 2015 | LEK PHARMACEUTICALS DD |
| 28923 | A | II | December 25, 2014 | ZHEJIANG HISUN PHARMACEUTICAL CO LTD |
| 28938 | A | II | January 27, 2015 | MSN ORGANICS PRIVATE LTD |
| 28984 | A | II | January 14, 2015 | CHANGZHOU PHARMACEUTICAL FACTORY |
| 29012 | A | II | February 13, 2015 | MICRO LABS LTD |
| 29065 | A | II | February 25, 2015 | RAKS PHARMA PVT LTD |
| 29568 | A | II | July 17, 2015 | WUHAN ZY PHARMACEUTICAL CO LTD |
| 31620 | A | II | March 24, 2017 | JIANGXI SYNERGY PHARMACEUTICAL CO LTD |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 60MG | 90MG | |
| Excipients used | mannitol (E421), calcium hydrogen phosphate dihydrate, sodium starch glycolate, hydroxypropyl-cellulose (E463), magnesium stearate (E470b) | mannitol (E421) (126.0MG), calcium hydrogen phosphate dihydrate (63.0MG), sodium starch glycolate (9.0MG), hydroxypropyl-cellulose (E463) (5.6MG), magnesium stearate (E470b) (3.0MG) | |
| Composition of coating material | hydroxypropyl methylcellulose, titanium dioxide, polyethylene glycol 400, ferric oxide black, and ferric oxide red | hydroxypropyl methylcellulose (9.0MG), titanium dioxide (1.7MG), talc (1.0MG), polyethylene-glycol 400 (0.6MG), and ferric oxide yellow (0.1MG) | |
| Composition of caspule shell | - | ||
| Pharmaceutical Development |
Because ticagrelor is a low soluble drug substance (not ionised in the physiological pH range) and exhibits a moderate intrinsic permeability, there is potentially a higher risk that changes in formulation and processing parameters can affect clinical performance, and this was taken into account during development. Moreover, this low aqueous solubility of ticagrelor leads to an increase of relevance of particle size. Nevertheless, extensive dissolution studies, including solubility studies in human intestinal fluids, were performed and suggest that ticagrelor may be less sensitive to process and formulation parameters than what would be expectedfrom its formal BCS classification. The compatibility of ticagrelor with a range of commonly used pharmaceutical excipients to develop an immediate release tablet, suitable for tablets has been investigated throughout development and the following were retained: mannitol (as a diluent), dibasic calcium phosphate (as a diluent), sodium starch glycollate (as a disintegrant), hydroxypropyl-cellulose (as a binder), magnesium stearate (as a lubricant), purified water (as a lubrication fluid), hypromellose (as a film former), titanium dioxide (as an opacifier), talc (as an opacifier and lubricant), polyethylene glycol (as a plasticiser), ferric oxide yellow (as a colouring agent) and purified water (as solvent). The choice and function of the excipients in the final formulation have been described and justified. A wet granulation process was chosen and the formulation was optimised using multivariate experiment design. Satisfactory discussion on the development of the formulation and the dissolution characteristics is provided in the dossier. Critical aspects that could affect the dissolution have sufficiently been discussed (e.g. polymorphic form, particle size, production parameters etc.). A quality by design approach was applied to the container closure system and three design space boundaries have been set: chemical compatibility, moisture ingress and the level of protection against UV light. |
||
| Manufacture of the product | Ticagrelor film-coated tablets are manufactured using conventional manufacturing techniques. The manufacturing process consists of 8 consecutive steps: 1) granulation including dry mixing; 2) wet granulation; 3) drying; 4) milling; 5) lubrication; 6) compression; 7) coating and 8) packaging. | ||
| Tablet / Capsule Image |
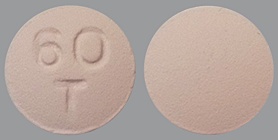
|

|
|
| Appearance | A round, biconvex, pink, film-coated tablet marked with “60” above “T” on one side | A round, biconvex, yellow, film-coated tablet marked with a “90” above “T” on one side | |
| Imprint code / Engraving / Debossment | Debossed with “60” above “T” on one side and plain on other side | Debossed with “90” above “T” on one side and plain on other side | |
| Score | no score | no score | |
| Color | PINK | YELLOW | |
| Shape | ROUND | ROUND | |
| Dimension | 8mm | 9mm | |
| Mfg by | AstraZeneca (EU) | ||
| Mfg for | - | ||
| Marketed by | AstraZeneca (US, EU) | ||
| Distributed by | AstraZeneca (US) | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N022433 | 2 | 6251910 | July 15, 2018 | Y | - | - | - | Download |
| N022433 | 2 | 7250419 | December 2, 2019 | Y | Y | U - 1171 | - | Download |
| N022433 | 2 | 7265124 | July 9, 2021 | Y | Y | U - 1171 | - | Download |
| N022433 | 2 | 8425934 | April 17, 2030 | - | Y | - | - | Download |
| N022433 | 2 | 6525060 | December 2, 2019 | DS | DP | U-1171 U-1860 U-1862 U-1863 | Y | Download |
| N022433 | 2 | RE46276 | December 2, 2019 | DS | DP | U-1935 U-1936 U-1937 U-1938 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.2% w/v Polysorbate 80 in water | 900 | 10, 20, 30, 45, 60 and 75 | June 25, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | BRILIQUE | Download |
| UK | BRILIQUE | Download |
| US | BRILINTA | Download |
| Exclusivity Code: Exclusivity Expiration is NS (New Strength): Sep 3, 2018 |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
