| Active Ingredient | TERIFLUNOMIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUBAGIO | (NDA) 202992 | SANOFI AVENTIS US | TABLET;ORAL | 7MG, 14MG | 7MG, 14MG (RS) | September 12, 2012 | September 12, 2017 | - | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
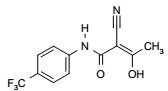 |
| Chemical Name | (Z)-2-Cyano-3-hydroxy-but-2-enoic acid-(4trifluoromethylphenyl)-amide |
| CAS No | 163451-81-8 |
| Molecular Formula | C12 H9F3N2O2 |
| Molecular Weight | 270.21 |
| Appearance | a white to almost white, odourless powder |
| Solubility | practically insoluble in water; sparingly soluble in acetone; and slightly soluble in polyethylene glycol, ethanol, acetonitrile and methylene chloride, very slightly soluble in isopropanol |
| Water Solubility | - |
| Polymorphism | The presence of polymorphs of teriflunomide has been evaluated using DSC and X-ray powder diffraction and recrystallization from different solvents and only one polymorphic form has been observed. In addition, single crystal X-ray diffraction analysis studies have demonstrated that teriflunomide in the solid state (crystalline phase) is only the Z-isomer. |
| pKa (Strongest Acidic) | - |
| pKa (Strongest Basic) | - |
| Log P | - |
| Identification | HPLC, IR |
| Degradation | The data obtained in stress studies show that teriflunomide in solid state is very stable, while in solution under neutral, acid or oxidative conditions degradation is observed. |
| Hygroscopic | non-hygroscopic |
| Photostability study | not sensitive to lights |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | Teriflunomide is synthesized in three main steps starting from 4-(Trifluoromethyl) aniline, cyanoacetic acid and acetic anhydride. Teriflunomide is then crystallized and jet-milled. The packaging of teriflunomide drug substance consists of double low-density polyethylene (LDPE) bags placed inside fibre drums or cardboard boxes. |
| Parameters | Details |
|---|---|
| Indications and Usage | AUBAGIO is indicated for the treatment of patients with relapsing forms of multiple sclerosis. |
| Dosage and Administration | The recommended dose of AUBAGIO is 7 mg or 14 mg orally once daily. AUBAGIO can be taken with or without food. |
| Mechanism of action | Teriflunomide, an immunomodulatory agent with anti-inflammatory properties, inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in de novo pyrimidine synthesis. The exact mechanism by which teriflunomide exerts its therapeutic effect in multiple sclerosis is unknown but may involve a reduction in the number of activated lymphocytes in CNS. |
| Absorption |
Teriflunomide is the principal active metabolite of leflunomide and is responsible for leflunomide’s activity in vivo. At recommended doses, teriflunomide and leflunomide result in a similar range of plasma concentrations of teriflunomide. Based on a population analysis of teriflunomide in healthy volunteers and MS patients, median t1/2 was approximately 18 and 19 days after repeated doses of 7 mg and 14 mg respectively. It takes approximately 3 months respectively to reach steady-state concentrations. The estimated AUC accumulation ratio is approximately 30 after repeated doses of 7 or 14 mg. Median time to reach maximum plasma concentrations is between 1 to 4 hours post-dose following oral administration of teriflunomide. |
| Food Effect | Food does not have a clinically relevant effect on teriflunomide pharmacokinetics. |
| Distribution | Teriflunomide is extensively bound to plasma protein (>99%) and is mainly distributed in plasma. The volume of distribution is 11 L after a single intravenous (IV) administration. |
| Metabolism | Teriflunomide is the major circulating moiety detected in plasma. The primary biotransformation pathway to minor metabolites of teriflunomide is hydrolysis, with oxidation being a minor pathway. Secondary pathways involve oxidation, N-acetylation and sulfate conjugation. |
| Elimination | Teriflunomide is eliminated mainly through direct biliary excretion of unchanged drug as well as renal excretion of metabolites. Over 21 days, 60.1% of the administered dose is excreted via feces (37.5%) and urine (22.6%). After an accelerated elimination procedure with cholestyramine, an additional 23.1% was recovered (mostly in feces). After a single IV administration, the total body clearance of teriflunomide is 30.5 mL/h. |
| Peak plasma time (Tmax) | 1 to 4 hours |
| Half life | median t1/2 was approximately 18 and 19 days after repeated doses of 7 mg and 14 mg respectively. |
| Bioavailability | - |
| Age, gender | In a population analysis, the clearance rate for teriflunomide is 23% less in females than in males. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 27860 | A | II | January 29, 2014 | MSN LABORATORIES PRIVATE LTD |
| 28898 | A | II | March 16, 2016 | ALEMBIC PHARMACEUTICALS LTD |
| 28991 | A | II | March 20, 2015 | GLENMARK PHARMACEUTICALS LTD |
| 29701 | A | II | January 10, 2015 | HONOUR LAB LTD |
| 29705 | A | II | September 29, 2015 | UNICHEM LABORATORIES LTD |
| 29802 | A | II | September 29, 2015 | MEGAFINE PHARMA P LTD |
| 29825 | A | II | September 18, 2015 | OPTIMUS DRUGS PRIVATE LTD [5-METHYL-ISOXAZOLE-4-CARBOXYLIC ACID (TERIFLUNOMIDE INTERMEDIATE)] |
| 29851 | A | II | October 21, 2015 | RAKS PHARMA PVT LTD |
| 29862 | A | II | December 30, 2015 | FORMOSA LABORATORIES INC |
| 30220 | A | II | March 10, 2016 | PHARMAZELL GMBH |
| 30296 | A | IV | February 16, 2016 | SYMRISE INC |
| 30297 | A | II | March 5, 2016 | BIOCON LTD |
| 30346 | A | II | March 7, 2016 | OLON SPA |
| 30414 | A | II | March 29, 2016 | EMCURE PHARMACEUTICALS LTD |
| 30426 | A | II | April 1, 2016 | CADILA HEALTHCARE LTD |
| 30491 | A | II | April 21, 2016 | INTAS PHARMACEUTICALS LTD |
| 30506 | A | II | April 29, 2016 | NATCO PHARMA LTD |
| 30978 | A | II | November 10, 2016 | TIANJIN WEIJIE PHARMACEUTICAL CO LTD |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 7MG | 14MG | |
| Excipients used | lactose monohydrate, corn starch, hydroxypropylcellulose, microcrystalline cellulose, sodium starch glycolate, and magnesium stearate | lactose monohydrate (76MG), corn starch (38MG), hydroxypropylcellulose (3.5MG), microcrystalline cellulose (10.5MG), sodium starch glycolate (7.5MG), and magnesium stearate (0.5MG) | |
| Composition of coating material | Hypromellose, titanium dioxide, talc, polyethylene glycol and indigo carmine aluminum lake, iron oxide yellow | Hypromellose (3.607MG), titanium dioxide (0.902MG), talc (0.271MG), polyethylene glycol (0.158MG ) and indigo carmine aluminum lake (0.62MG) | |
| Composition of caspule shell | - | ||
| Pharmaceutical Development |
The development studies of the tablet focused on physicochemical properties of the drug substance, which were identified as potentially having a higher impact on drug product performance. These properties were particle size, water content, stability and purity. Particle size was initially identified as potentially impacting dissolution and thus bioavailability because teriflunomide is a BCS Class 2 active substance (high permeability, low solubility). To investigate whether particle size had an impact on bioavailability, a bioequivalence study with formulations manufactured with milled versus unmilled, sieved drug was performed. This study demonstrated that the particle size of the drug substance (within the range studied) does not have an impact on bioavailability. In addition, it was demonstrated that particle size has no impact on content uniformity. Colloidal anhydrous silica was removed from the tablet core formulation, as it was shown that it had an effect on the stability of teriflunomide within the drug product, promoting the formation of one of the degradation products. In addition, the film-coating thickness was slightly increased. Since colloidal anhydrous silica may affect the dissolution, and thus absorption and efficacy, a bioequivalence study was conducted. |
||
| Manufacture of the product | The manufacturing process for teriflunomide commercial film-coated tablets is a standard wet granulation process involving conventional mixing, fluid–bed granulation, drying, sieving, mixing and lubrication, tableting and film-coating. | ||
| Tablet / Capsule Image |

|
||
| Appearance | a very light greenish-bluish grey to pale greenish-blue, hexagonal film-coated tablet with dose strength “7” imprinted on one side and engraved with the corporate logo on other side. | a pale blue to pastel blue, pentagonal film-coated tablet with the dose strength, “14” imprinted on one side and engraved with the corporate logo on the other side. | |
| Imprint code / Engraving / Debossment | “7” imprinted on one side and engraved with the corporate logo on other side. | “14” imprinted on one side and engraved with the corporate logo on the other side. | |
| Score | no score | no score | |
| Color | a very light greenish-bluish grey to pale greenish-blue | a pale blue to pastel blue | |
| Shape | hexagonal | pentagonal | |
| Dimension | 7mm | 8mm | |
| Mfg by | sanofi group (EU) | ||
| Mfg for | - | ||
| Marketed by | Genzyme Corporation (US), sanofi-aventis group (EU) | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N202992 | 1 | 6794410 | April 15, 2022 | - | - | U - 1285 | - | Download |
| N202992 | 1 | 8802735 | September 14, 2030 | - | Y | - | - | Download |
| N202992 | 1 | 9186346 | February 4, 2034 | - | - | U - 1786 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.05 M Phosphate Buffer, pH 6.8 | 1000 | 5, 10, 15, 20, 30 and 45 | May 15, 2014 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | AUBAGIO 14MG Tablet | Download |
| UK | AUBAGIO 14MG Tablet | Download |
| US | AUBAGIO | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
