| Active Ingredient | TALAZOPARIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TALZENNA | 211651 | PFIZER INC | CAPSULE;ORAL | 0.25MG, 1 MG | 1 MG | October 16, 2018 | October 16, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
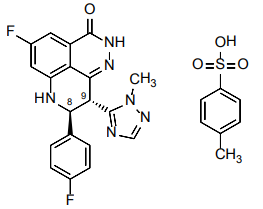 |
| Chemical Name | (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one 4-methylbenzenesulfonate (1:1) |
| CAS No | Tosylate salt: 1373431-65-2; Free base: 1207456-01-6 |
| Molecular Formula | C26H15F2N6O4S (tosylate salt); C19H14F2N6O4 (free base) |
| Molecular Weight | 552.56 (tosylate salt); 380.35 (free base) |
| Appearance | White to yellow solid |
| Solubility | It has limited solubility in aqueous solutions, and the solubility increases as the pH increases. It is freely soluble in DMS, DMA and DMF. |
| Water Solubility | - |
| Polymorphism | Talazoparib tosylate exists as a single crystal form and no other polymorphs have been observed through extensive screening studies during development including conditions covering the solvent compositions used in the final isolation process |
| pKa (Strongest Acidic) | 9.48 |
| pKa (Strongest Basic) | 1.66 |
| Log P | 1.6 |
| Identification | - |
| Degradation | - |
| Hygroscopic | Non-hygroscopic |
| Photostability study | - |
| Melting Point | 326oC |
| BCS Class | - |
| Manufacture of API | It is synthesized in a 6-step chemical synthesis. Talazoparib Tosylate is manufactured by 6-step chemical synthesis. |
| Parameters | Details |
|---|---|
| Indications and Usage | TALZENNA is a poly (ADP-ribose) polymerase (PARP) inhibitor indicated for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) HER2-negative locally advanced or metastatic breast cancer. Select patients for therapy based on an FDA-approved companion diagnostic for TALZENNA. |
| Dosage and Administration |
The recommended dose of TALZENNA is 1 mg taken as a single oral daily dose, with or without food. • Patients should be treated until disease progression or unacceptable toxicity occurs. • For adverse reactions, consider dosing interruption or dose reduction. • For patients with moderate renal impairment (CLcr 30 - 59 mL/min), the recommended dose of TALZENNA is 0.75 mg once daily. |
| Mechanism of action |
Talazoparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1 and PARP2,which play a role in DNA repair. In vitro studies with cancer cell lines that harbored defects in DNA repair genes, including BRCA 1 and 2, have shown that talazoparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, decreased cell proliferation, and apoptosis. Talazoparib anti-tumor activity was observed in human patient-derived xenograft breast cancer tumor models that expressed mutated or wild-type BRCA 1 and 2. |
| Absorption |
After oral administration of 1 mg TALZENNA once daily in patients, the recommended dose, the geometric mean [% coefficient of variation (CV%)] of AUC and maximum observed plasma concentration (Cmax) of talazoparib at steady-state was 208 (37%) ng.hr/mL and 16.4 (32%) ng/mL, respectively. The pharmacokinetics (PK) of talazoparib is linear from 0.025 mg to 2 mg (2 times the recommended dose). The median accumulation ratio of talazoparib following repeated oral administration of 1 mg once daily was in the range of 2.3 to 5.2.Talazoparib plasma concentrations reached steady-state within 2 to 3 weeks. Absorption Following oral administration of talazoparib, the median time to Cmax (Tmax) was generally between 1 to 2 hours after dosing. |
| Food Effect | Following a single oral dose of 0.5 mg TALZENNA with high-fat, high-calorie food (approximately 800 to 1000 calories with 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively), the mean Cmax of talazoparib was decreased by 46%, the median Tmax was delayed from 1 to 4 hours, and AUCinf was not affected. |
| Distribution | The mean apparent volume of distribution of talazoparib is 420 L. In vitro, protein binding of talazoparib is 74% and is independent of talazoparib concentration. |
| Metabolism | Talazoparib undergoes minimal hepatic metabolism. The identified metabolic pathways of talazoparib in humans include mono-oxidation, dehydrogenation, cysteine conjugation of mono-desfluoro-talazoparib, and glucuronide conjugation. |
| Elimination |
Elimination The mean terminal plasma half-life (±standard deviation) of talazoparib is 90 (±58) hours, and the mean apparent oral clearance (inter-subject variability) is 6.45 L/h (31.1%) in cancer patients. Excretion Excretion of talazoparib in urine was the major route of elimination. Approximately 68.7% (54.6% unchanged) of the total administered radioactive dose [14C]talazoparib was recovered in urine, and 19.7% (13.6%unchanged) was recovered in feces. |
| Peak plasma time (Tmax) | 1 to 2 hours |
| Half life | 90 (±58) hours |
| Bioavailability | _ |
| Age, gender | Age (18 to 88 years), sex, race (361 White, 41 Asian, 16 Black, 9 Others, and 63 Not Reported), and body weight (36 to 162 kg) had no clinically relevant effect on the PK of talazoparib. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||
|---|---|---|---|
| Strength | 0.25 MG | 1 MG | |
| Excipients used | Silicified microcrystalline cellulose (sMCC) | ||
| Composition of coating material | - | ||
| Composition of caspule shell |
HPMC, yellow iron oxide, red iron oxide and titanium dioxide; and the printing ink contains shellac, black iron oxide, potassium hydroxide, ammonium hydroxide, and propylene glycol. |
||
| Pharmaceutical Development | TALZENNA capsules for oral use are available as a 0.25 mg hard hypromellose (HPMC) capsule that contains 0.363 mg talazoparib tosylate equivalent to 0.25 mg talazoparib free base or as a 1 mg HPMC capsule that contains 1.453 mg talazoparib tosylate equivalent to 1 mg talazoparib free base. | ||
| Manufacture of the product | Updated soon | ||
| Tablet / Capsule Image | |||
| Appearance | Ivory cap (printed with “Pfizer” in black) and a white body (printed with “TLZ 0.25” in black). | Light red cap (printed with “Pfizer” in black) and a white body (printed with “TLZ 1” in black). | |
| Imprint code / Engraving / Debossment | "Pfizer” and “TLZ 0.25” | "Pfizer” and “TLZ 1” | |
| Score | No score | No score | |
| Color | Ivory cap and white body | Light red cap and white body | |
| Shape | Capsule | Capsule | |
| Dimension | - | 14 mm | |
| Mfg by | - | ||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by | pfizer Labs | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N211651 | 1 | 8012976 | October 19, 2029 | DS | DP | - | - | Download |
| N211651 | 1 | 8420650 | July 27, 2029 | DS | DP | - | - | Download |
| N211651 | 1 | 8735392 | October 20, 2031 | DS | DP | - | - | Download |
| N211651 | 1 | 9820985 | July 27, 2029 | - | - | U-2437 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II | 75 | 0.01 N HCl with 0.2% SDS | 500 | Q in 30 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
