| Active Ingredient | TAFAMIDIS |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VYNDAMAX | (NDA): 212161 | FOLDRX PHARMS | CAPSULE;ORAL | 61 MG | 61 MG | May 3, 2019 | May 3, 2024 | May 3, 2026 | Type 2 - New Active Ingredient | STANDARD; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
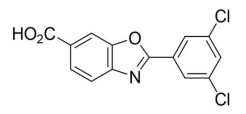 |
| Chemical Name | 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid |
| CAS No | 594839-88-0 |
| Molecular Formula | C14H7Cl2NO3 |
| Molecular Weight | 308.12 g/mol |
| Appearance | - |
| Solubility | Poorly soluble pH independetn solubility/insolubility |
| Water Solubility | Poorly soluble in water |
| Polymorphism | Different polymorphic form. Form 1, Form 2, Form 4, Form 6. Form 1 could be used in drug product |
| pKa (Strongest Acidic) | 3.73 (Drug bank) |
| pKa (Strongest Basic) | - |
| Log P | 3.91 (Drug bank) |
| Identification | - |
| Degradation | - |
| Hygroscopic | Non Hygroscopic |
| Photostability study | Not Light sensitive |
| Melting Point | - |
| BCS Class | IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | VYNDAQEL and VYNDAMAX are transthyretin stabilizers indicated for the treatment of the cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization |
| Dosage and Administration |
The recommended dosage is either: • VYNDAQEL 80 mg orally once daily, or • VYNDAMAX 61 mg orally once daily |
| Mechanism of action | Tafamidis is a selective stabilizer of TTR. Tafamidis binds to TTR at the thyroxine binding sites, stabilizing the tetramer and slowing dissociation into monomers, the rate-limiting step in the amyloidogenic process. |
| Absorption |
No clinically significant differences in steady state Cmax and area under the plasma concentration over time curve (AUC) of tafamidis were observed for VYNDAMAX 61-mg capsule compared to VYNDAQEL administered as four 20-mg capsules. Tafamidis exposure increases proportionally over single (up to 480 mg) or multiple (up to 80 mg) (1 to 6 times the approved recommended dosage) once daily dosing.The apparent clearance were similar after single and repeated administration of VYNDAQEL 80 mg. Absorption Median tafamidis peak concentrations occurred within 4 hours following dosing |
| Food Effect | No clinically significant differences in the pharmacokinetics of tafamidis were observed following administration of a high fat, high calorie meal. |
| Distribution | The apparent steady state volume of distribution of tafamidis is approximately 18.5 liters. Plasma protein binding of tafamidis is >99% in vitro. Tafamidis primarily binds to TTR |
| Metabolism | The metabolism of tafamidis has not been fully characterized. However, glucuronidation has been observed. |
| Elimination |
The mean half-life of tafamidis is approximately 49 hours. The apparent oral clearance of tafamidis is 0.263 L/hr. The degree of drug accumulation at steady state after repeated tafamidis daily dosing is approximately 2.5-fold greater than that observed after a single dose. Excretion After a single oral dose of tafamidis meglumine 20 mg, approximately 59% of the dose was recovered in feces (mostly as the unchanged drug) and approximately 22% of the dose was recovered in urine (mostly as the glucuronide metabolite). |
| Peak plasma time (Tmax) | 4 hours |
| Half life | Approximately 49 hours |
| Bioavailability | - |
| Age, gender | No clinically significant differences in the pharmacokinetics of tafamidis were observed based on age,race/ethnicity (Caucasian and Japanese) or renal impairment. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 61 MG |
| Excipients used |
Ammonium hydroxide 28%, butylated hydroxytoluene, ethyl alcohol, gelatin, glycerin, iron oxide (red), isopropyl alcohol, polyethylene glycol 400, polysorbate 20, povidone (K-value 90), polyvinyl acetate phthalate, propylene glycol, purified water, sorbitol, and titanium dioxide. |
| Composition of coating material | NA |
| Composition of caspule shell | NA |
| Pharmaceutical Development |
Tafamidis 61-mg soft gelatin capsule for oral use contains a white to pink colored suspension of tafamidis 61 mg. |
| Manufacture of the product | - |
| Tablet / Capsule Image | |
| Appearance | Soft gelatin capsules are reddish brown, opaque, oblong, and printed with “VYN 61” in white |
| Imprint code / Engraving / Debossment | Printed with “VYN 61” in white |
| Score | No Score |
| Color | Reddish brown, Opaque |
| Shape | Oblong |
| Dimension | Size 9.5 soft gelatin capsule |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by | Pfizer Labs, division of Pfize Inc, NY, NY 10017 |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N212161 | 1 | 7214695 | April 27, 2024 | DS | DP | - | - | Download |
| N212161 | 1 | 7214696 | December 19, 2023 | - | - | U-2524 | - | Download |
| N212161 | 1 | 9770441 | August 31, 2035 | DS | DP | U-2524 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | Tier 1: 0.05 M Sodium Phosphate , pH 6.8 with 1.0% tween 80 Tier 2: 0.05 M Sodium phosphate buffer pH 6.8 with 1.0% Tween 80 and NMT 2000 Units of protease activity / L | 900 mL | Q point in 30 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
