| Active Ingredient | TADALAFIL |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIALIS | NDA#021368 | LILLY | TABLET;ORAL | 2.5MG, 5MG, 10MG, 20MG | 2.5MG, 5MG, 10MG, 20MG | November 21, 2003 | - | - | 1 New molecular entity (NME) | STANDARD | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
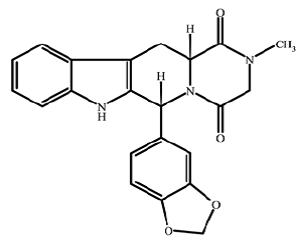 |
| Chemical Name | Pyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione,6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-,(6R-trans)-(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-[3,4-(methylenedioxy)phenyl]-pyrazino[1',2':1,6] pyrido [3,4-b]indole-1,4-dione |
| CAS No | 171596-29-5 |
| Molecular Formula | C22H19N3O4 |
| Molecular Weight | 389.41 |
| Appearance | crystalline solid |
| Solubility | It is practically insoluble in water and very slightly soluble in ethanol. It does not possess any ionisable groups in the pH range of 1-11 and, subsequently, does not demonstrate any changes in solubility in aqueous buffers in that range. It is freely soluble only in solvents such as dimethylsulfoxide and dimethylformamide. |
| Water Solubility | 0.25 mg/mL |
| Polymorphism | This molecule has 2 chiral centres, and therefore four different stereoisomers may be found. The molecule obtained in the process described is in the RR form.. Crystallization studies show that tadalafil does not exhibit polymorphism. |
| pKa (Strongest Acidic) | 15.17 |
| pKa (Strongest Basic) | -4.2 |
| Log P | 1.7 |
| Identification | IR, HPLC |
| Degradation | No degradation or physical changes were observed in tadalafil at its solid state under heat, humidity and light exposure. However, degradation was observed in solutions and suspensions of the active substance at different pH values, light and oxidative conditions. |
| Hygroscopic | Hygroscopic |
| Photostability study | - |
| Melting Point | 301-302 °C |
| BCS Class | II |
| Manufacture of API | Tadalafil drug substance is synthesised by a 3-step process, with purifications following each step. After the final drying, the resulting solid is milled to meet the established particle size specification.” |
| Parameters | Details |
|---|---|
| Indications and Usage | CIALIS® is indicated for the treatment of erectile dysfunction (ED). CIALIS is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH). Limitation of Use: If CIALIS is used with finasteride to initiate BPH treatment, such use is recommended for up to 26 weeks because the incremental benefit of CIALIS decreases from 4 weeks until 26 weeks, and the incremental benefit of CIALIS beyond 26 weeks is unknown. |
| Dosage and Administration |
The recommended starting dose for Erectile Dysfunction 10 mg, taken prior to anticipated sexual activity. The dose may be increased to 20 mg or decreased to 5 mg, based on individual efficacy and tolerability. The maximum recommended dosing frequency is once per day in most patients. The recommended dose of CIALIS for once daily use in Benign Prostatic Hyperplasia is 5 mg, taken at approximately the same time every day. CIALIS may be taken without regard to food. Refer FDA Label for more information. |
| Mechanism of action |
CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle. This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cGMP in smooth muscle cells. Cyclic GMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum. The inhibition of phosphodiesterase type 5 (PDE5) enhances erectile function by increasing the amount of cGMP. Tadalafil inhibits PDE5. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 by tadalafil has no effect in the absence of sexual stimulation. The effect of PDE5 inhibition on cGMP concentration in the corpus cavernosum and pulmonary arteries is also observed in the smooth muscle of the prostate, the bladder and their vascular supply. The mechanism for reducing BPH symptoms has not been established. Studies in vitro have demonstrated that tadalafil is a selective inhibitor of PDE5. PDE5 is found in the smooth muscle of the corpus cavernosum, prostate, and bladder as well as in vascular and visceral smooth muscle, skeletal muscle, urethra, platelets, kidney, lung, cerebellum, heart, liver, testis, seminal vesicle, and pancreas. |
| Absorption |
Over a dose range of 2.5 to 20 mg, tadalafil exposure (AUC) increases proportionally with dose in healthy subjects. Steady-state plasma concentrations are attained within 5 days of once per day dosing and exposure is approximately 1.6-fold greater than after a single dose. After single oral-dose administration, the maximum observed plasma concentration (Cmax) of tadalafil is achieved between 30 minutes and 6 hours (median time of 2 hours). Absolute bioavailability of tadalafil following oral dosing has not been determined. |
| Food Effect | The rate and extent of absorption of tadalafil are not influenced by food; thus CIALIS may be taken with or without food. |
| Distribution |
The mean apparent volume of distribution following oral administration is approximately 63 L, indicating that tadalafil is distributed into tissues. At therapeutic concentrations, 94% of tadalafil in plasma is bound to proteins. Less than 0.0005% of the administered dose appeared in the semen of healthy subjects. |
| Metabolism | Tadalafil is predominantly metabolized by CYP3A4 to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation to form the methylcatechol and methylcatechol glucuronide conjugate, respectively. The major circulating metabolite is the methylcatechol glucuronide. Methylcatechol concentrations are less than 10% of glucuronide concentrations. In vitro data suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations. |
| Elimination | The mean oral clearance for tadalafil is 2.5 L/hr and the mean terminal half-life is 17.5 hours in healthy subjects. Tadalafil is excreted predominantly as metabolites, mainly in the feces (approximately 61% of the dose) and to a lesser extent in the urine (approximately 36% of the dose). |
| Peak plasma time (Tmax) | 2 hours |
| Half life | 17.5 hours |
| Bioavailability | - |
| Age, gender | Healthy male elderly subjects (65 years or over) had a lower oral clearance of tadalafil, resulting in 25% higher exposure (AUC) with no effect on Cmax relative to that observed in healthy subjects 19 to 45 years of age. No dose adjustment is warranted based on age alone. However, greater sensitivity to medications in some older individuals should be considered. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 20640 | A | II | June 15, 2007 | MYLAN LABORATORIES LTD |
| 20884 | A | II | September 26, 2007 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 20962 | A | II | October 22, 2007 | ORCHID PHARMA LTD |
| 21209 | A | II | January 7, 2008 | DR REDDYS LABORATORIES LTD |
| 22530 | A | II | February 16, 2009 | GLENMARK PHARMACEUTICALS LTD |
| 22707 | A | II | March 31, 2009 | ALEMBIC PHARMACEUTICALS LTD |
| 22771 | I | II | May 13, 2009 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 24206 | A | II | September 28, 2010 | SMS PHARMACEUTICALS LTD |
| 24590 | A | II | January 31, 2011 | ZAKLADY FARMACEUTYCZNE POLPHARMA SA |
| 25355 | I | II | October 5, 2011 | AMI LIFE SCIENCES PRIVATE LTD |
| 25513 | A | II | November 15, 2011 | MSN ORGANICS PRIVATE LTD |
| 25694 | A | II | January 18, 2012 | AUROBINDO PHARMA LTD |
| 25815 | A | II | February 22, 2012 | APOTEX PHARMACHEM INDIA PVT LTD |
| 26139 | A | II | April 5, 2013 | MACLEODS PHARMACEUTICALS LTD |
| 26786 | A | II | February 8, 2013 | MSN ORGANICS PRIVATE LTD |
| 27246 | A | II | June 28, 2013 | JUBILANT GENERICS LTD |
| 27479 | A | II | September 27, 2013 | ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD |
| 29027 | A | II | February 3, 2015 | UNICHEM LABORATORIES LTD |
| 29316 | A | II | June 24, 2015 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 29595 | A | II | September 30, 2015 | GLENMARK PHARMACEUTICALS LTD |
| 29765 | A | II | December 4, 2015 | HONOUR LAB LTD |
| 30608 | A | II | June 10, 2016 | AMOLI ORGANICS PVT LTD |
| 30860 | A | II | September 5, 2016 | QILU TIANHE PHARMACEUTICAL CO LTD |
| Parameters | Details | ||||
|---|---|---|---|---|---|
| Strength | 2.5MG | 5MG | 10MG | 20MG | |
| Excipients used |
croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, talc |
||||
| Composition of coating material | lactose monohydrate, hypromellose, triacetin, titanium dioxide (E171), iron oxide yellow (E172), iron oxide red (E172), talc. |
lactose monohydrate, hypromellose, triacetin, titanium dioxide (E171), iron oxide yellow (E172), talc. |
|||
| Composition of caspule shell | - | ||||
| Pharmaceutical Development |
The development pharmaceutics have taken into consideration the physicochemical characteristics of the active drug substance such as poor aqueous solubility, hygroscopic properties, stability, particle size, and biopharmaceutical issues such as dissolution rate. The formulation contains stable, milled tadalafil drug substance and incorporated into a wet granulation to consistently produce tablets with good homogeneity and the desired dissolution characteristics. To achieve a dosage form with a rapid therapeutic onset, the excipients included in the formulation were selected and adjusted to promote rapid absorption. This combination of ingredients has produced a material (free-flowing, easily wetted and compressible) as well as tablets that possess the desired physical characteristics of low friability, acceptable hardness and rapid disintegration. |
||||
| Manufacture of the product |
The process for the manufacturing of the finished product follows conventional pharmaceutical practices, which includes aqueous wet granulation, sizing, and drying steps of tadalafil with hydrophilic excipients, followed by dry sizing of the granulate and then blending with additional excipients. The final blend is compressed into tablets, which are subsequently coated. Validation of the manufacturing process was performed on 3 full-scale batches. For each one of those batches, specific conditions at each stage were investigated (mixing speeds, times, screen size, temperature, rotation speeds, etc.). Results indicate that the process is adequately validated and controlled. |
||||
| Tablet / Capsule Image |

|

|

|

|
|
| Appearance | Almond-shaped, tablets debossed with “C 2 1/2” on one side and plain on other side. | Almond-shaped, tablets debossed with “C 5” on one side and plain on other side. | Almond-shaped, tablets debossed with “C 10” on one side and plain on other side. | Almond-shaped, tablets debossed with “C 20” on one side and plain on other side. | |
| Imprint code / Engraving / Debossment | "C 2 1/2" | "C 5" | "C 10" | "C 20" | |
| Score | no score | no score | no score | no score | |
| Color | Yellow | Yellow | Yellow | Yellow | |
| Shape | OVAL (Almond) | OVAL (Almond) | OVAL (Almond) | OVAL (Almond) | |
| Dimension | - | - | 11mm | 12mm | |
| Mfg by | Eli Lilly and Company (US, EU) | ||||
| Mfg for | Eli Lilly and Company (US, EU) | ||||
| Marketed by | Eli Lilly and Company (US, EU) | ||||
| Distributed by | Eli Lilly and Company (US, EU) | ||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021368 | 4 | 5859006 | 21-Nov-17 | DS | DP | - | - | Download |
| N021368 | 4 | 6140329 | 11-Jul-16 | - | DP | U-155 | - | Download |
| N021368 | 4 | 6821975 | 19-Nov-20 | DS | DP | U-533 U-614 | - | Download |
| N021368 | 4 | 6943166 | 26-Apr-20 | - | - | U-155 | - | Download |
| N021368 | 4 | 7182958 | 26-Apr-20 | - | DP | U-155 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.5% Sodium Lauryl Sulfate | 1000 | 10, 20, 30 and 45 | January 26, 2006 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | CIALIS | Download |
| UK | CIALIS | Download |
| US | ALEMBIC PHARMS LTD (ANDA #204809) | |
| US | AUROBINDO PHARMA LTD (ANDA #206285) | |
| US | CIALIS | Download |
| US | SUN PHARMA GLOBAL (ANDA #208934) | |
| US | SYNTHON PHARMS (ANDA #200630) | |
| US | WATSON LABS INC (ANDA #205885) |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
