| Active Ingredient | STIRIPENTOL |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DIACOMIT | 206709 | BIOCODEX SA | CAPSULE;ORAL | 250MG, 500 MG | 500 MG | August 20, 2018 | August 20, 2023 | August 20, 2025 | Type 1 - New Molecular Entity | PRIORITY; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
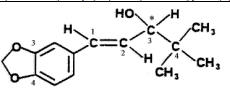 |
| Chemical Name | 4,4-dimethyl-1-[3,4-(methylendioxyphenyl)-1-pentene-3-ol |
| CAS No | 49763-96-4 |
| Molecular Formula | C14H18O3 |
| Molecular Weight | 234.3 |
| Appearance | White to pale yellow crystalline powder with a bitter taste |
| Solubility | Sparingly soluble in chloroform, and soluble in acetone, ethanol,ether, acetonitrile, and dichloromethane |
| Water Solubility | Practically insoluble in water (at 25°C) |
| Polymorphism | Stiripentol has not been observed to exhibit polymorphism. |
| pKa (Strongest Acidic) | 14.2 |
| pKa (Strongest Basic) | -3.1 |
| Log P | 2.94 |
| Identification | HPLC, IR and colour reaction |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 75°C |
| BCS Class | II |
| Manufacture of API | Stiripentol is synthesised in a simple two-stage process beginning with an aldol condensation and subsequent reduction of the resulting ketone. This condensation step is reported in literature to largely yield the trans isomer, whilst the reduction of the ketone to stiripentol is non-selective and yields the racemate. Confirmation that the trans isomer is initially formed has been provided by spectroscopic evidence. Very low levels of the cis-isomer have been detected in 10 batches. Satisfactory spectroscopic evidence has been provided to confirm the structure of the active substance which is routinely produced according to the defined synthetic process. The absence of polymorphism has been satisfactorily addressed by means of X-ray powder diffraction and DTA studies. |
| Parameters | Details |
|---|---|
| Indications and Usage | DIACOMIT is indicated for the treatment of seizures associated with Dravet syndrome in patients 2 years of age and older taking clobazam. There are no clinical data to support the use of DIACOMIT as monotherapy in Dravet syndrome |
| Dosage and Administration |
The dosage of DIACOMIT is 50 mg/kg/day, administered by mouth in 2 or 3 divided doses. • Reduce dose or discontinue dose gradually. • Capsules must be swallowed whole with a glass of water during a meal.Capsules should not be broken or opened. • Powder for suspension should be mixed in a glass of water and should be taken immediately after mixing during a meal |
| Mechanism of action | The mechanism by which DIACOMIT exerts its anticonvulsant effect in humans is unknown.Possible mechanisms of action include direct effects mediated through the gamma-aminobutyric acid (GABA)A receptor and indirect effects involving inhibition of cytochrome P450 activity with resulting increase in blood levels of clobazam and its active metabolite. |
| Absorption |
The following pharmacokinetic properties of stiripentol have been found in studies in adult healthy volunteers and adult patients. Systemic exposure of stiripentol increases in a greater than dose proportional manner from 500 mg to 2000 mg. Absorption: The median time to stiripentol peak plasma concentration is 2 to 3 hours. |
| Food Effect | _ |
| Distribution | Protein binding of stiripentol is 99% |
| Metabolism | On the basis of in vitro studies, the main liver cytochrome P450 (CYP) isoenzymes involved in metabolism are considered to be CYP1A2, CYP2C19, and CYP3A4. |
| Elimination | The elimination half-life of stiripentol ranges from 4.5 to 13 hours, increasing with doses of 500 mg, 1000 mg and 2000 mg. |
| Peak plasma time (Tmax) | 2 to 3 hours |
| Half life | 4.5 to 13 hours |
| Bioavailability | - |
| Age, gender | The effect of age (≥ 65 years), race, renal and hepatic impairment on stiripentol pharmacokinetics is unknown [see Use in Specific Populations (85, 8.6, 8.7)]. Sex does not have a clinically significant effect on the pharmacokinetics of DIACOMIT |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||
|---|---|---|---|
| Strength | 250 MG | 500 MG | |
| Excipients used | Magnesium stearate, povidone, sodium starch glycolate (0.16 mg sodium per capsule) |
Magnesium stearate, povidone, sodium starch glycolate (0.32 mg sodium per capsule. ) |
|
| Composition of coating material | - | ||
| Composition of caspule shell | Erythrosine,gelatin, indigotine, titanium dioxide. | Gelatin, titanium dioxide | |
| Pharmaceutical Development |
DIACOMIT capsules contain 250 mg (size 2: pink) or 500 mg (size 0: white). Studies have naturally focussed on the solid-state properties of the active substance, e.g. particle size control and polymorphism. Solid state active-excipient compatibility studies with a range of excipients under elevated temperatures has been investigated and no evidence of incompatibility was noted with the excipients finally selected. Starch and PVP were tested as binders and PVP selected as it yielded granules with good flow with minimal variation in density. Sodium starch glycolate is added as disintegrant and is incorporated intra- (1%) and extra-granularly (0.5%). Magnesium stearate added extra-granularly is used as lubricant at a level of 0.5%. Excipients used are standard pharmacopoeial ingredients for solid-dose preparations.Stiripentol is practically insoluble in water, but is well absorbed following oral administration. Thus since the particle size is controlled, the rate determining step is likely to be dissolution. Originally a hydroalcoholic dissolution medium was developed for routine quality control although this was replaced with an aqueous sodium laurilsulphate solution which gives better discriminatory power between batches with different active substance particle sizes |
||
| Manufacture of the product | The manufacturing process is relatively straightforward and involves standard pharmaceutical unit operations: mixing, granulation, tray oven drying, screening, extra-granular blending and encapsulation before final packaging. The process and equipment have been adequately described.In-process controls are satisfactory for the processes described. | ||
| Tablet / Capsule Image | |||
| Appearance | Size 2, pink, and imprinted with “Diacomit” and “250mg” | Size 0, white, and imprinted with “Diacomit” and “500mg” | |
| Imprint code / Engraving / Debossment | Imprinted with “Diacomit” and “250mg” | Imprinted with “Diacomit” and “500mg” | |
| Score | No score | No score | |
| Color | Pink | White | |
| Shape | Capsule | Capsule | |
| Dimension | Size 2 (15mm) | Size 0 ( 18mm) | |
| Mfg by |
BIOCODEX 1, avenue Blaise Pascal 60000 BEAUVAIS France |
||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II | 75 RPM | 1.2% solution adjusted to pH 4.5 | 900 mL | Q in 20 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | DIACOMIT | Download |
| UK | DIACOMIT | |
| US | DIACOMIT | Download |
| Product shelf life- 36 month at 25°C/60% RH |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
