| Active Ingredient | SEMAGLUTIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RYBELSUS | 213051 | NOVO | TABLET;ORAL | 3MG, 7MG, 14MG | 14MG | September 20, 2019 | December 05,2022 (NP) | _ | Type 3 - New Dosage Form | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
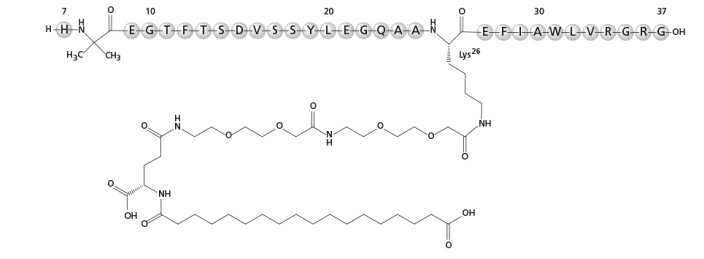 |
| Chemical Name | Modified analogue of human GLP-1 [7-37] peptide |
| CAS No | 910463-68-2 |
| Molecular Formula | C187H291N45O59 |
| Molecular Weight | 4113.58 g/mol |
| Appearance | White to almost white hygroscopic powder |
| Solubility | - |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | 2.74 |
| pKa (Strongest Basic) | 12.26 |
| Log P | -18 |
| Identification | - |
| Degradation | - |
| Hygroscopic | Hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | BCS Class IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | RYBELSUS is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use : Not recommended as first-line therapy for patients inadequately controlled on diet and exercise. Has not been studied in patients with a history of pancreatitis. Not for treatment of type 1 diabetes mellitus. |
| Dosage and Administration |
Instruct patients to take RYBELSUS at least 30 minutes before the first food, beverage, or other oral medications of the day with no more than 4 ounces of plain water only. Waiting less than 30 minutes, or taking with food, beverages (other than plain water) or other oral medications will lessen the effect of RYBELSUS. Waiting more than 30 minutes to eat may increase the absorption of RYBELSUS. Swallow tablets whole. Do not cut, crush, or chew tablets. Start RYBELSUS with 3 mg once daily for 30 days. After 30 days on the 3 mg dose, increase the dose to 7 mg once daily. Dose may be increased to 14 mg once daily if additional glycemic control is needed after at least 30 days on the 7 mg dose. See the Full Prescribing Information for instructions on switching between OZEMPIC® and RYBELSUS. |
| Mechanism of action | Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1. GLP-1 is a physiological hormone that has multiple actions on glucose, mediated by the GLP-1 receptors. The principal mechanism of protraction resulting in the long half-life of semaglutide is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilized against degradation by the DPP-4 enzyme. Semaglutide reduces blood glucose through a mechanism where it stimulates insulin secretion and lowers glucagon secretion, both in a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase. |
| Absorption |
Semaglutide is co-formulated with salcaprozate sodium which facilitates the absorption of semaglutide after oral administration. The absorption of semaglutide predominantly occurs in the stomach. Population pharmacokinetics (PK) estimated semaglutide exposure to increase in a dose-proportional manner. In patients with type 2 diabetes, the mean population-PK estimated steady-state concentrations following once daily oral administration of 7 and 14 mg semaglutide were approximately 6.7 nmol/L and 14.6 nmol/L, respectively. Following oral administration, maximum concentration of semaglutide is reached 1 hour post-dose. Steady-state exposure is achieved following 4-5 weeks administration. Population-PK estimated absolute bioavailability of semaglutide to be approximately 0.4%-1%, following oral administration. |
| Food Effect | - |
| Distribution | The estimated volume of distribution of semaglutide following oral administration in healthy subjects is approximately 8 L. Semaglutide is extensively bound to plasma albumin (>99%). |
| Metabolism | The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain. |
| Elimination |
Elimination : With an elimination half-life of approximately 1 week, semaglutide is present in the circulation for about 5 weeks after the last dose. The clearance of semaglutide following oral administration in healthy subjects is approximately 0.04 L/h. Excretion : The primary excretion routes of semaglutide-related material are via the urine and feces. Approximately 3% of the absorbed dose is excreted in the urine as intact semaglutide. |
| Peak plasma time (Tmax) | 1 hour post-dose. Steady-state exposure is achieved following 4-5 weeks administration. |
| Half life | Approximately 1 week |
| Bioavailability | 0.4%-1% |
| Age, gender | Based on a population pharmacokinetic analysis, age, sex, race, ethnicity, upper GI disease, and renal impairment do not have a clinically meaningful effect on the pharmacokinetics of semaglutide. The exposure of semaglutide decreases with an increase in body weight. However, RYBELSUS doses of 7 mg and 14 mg provide adequate systemic exposure over the body weight range of 40-188 kg evaluated in the clinical trials. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 35712 | A | II | March 18, 2021 | ZHEJIANG PEPTITES BIOTECH CO LTD SEMAGLUTIDE |
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 3MG | 7MG | 14MG | |
| Excipients used | Magnesium stearate, microcrystalline cellulose, povidone and salcaprozate sodium (SNAC) | |||
| Composition of coating material | NA | |||
| Composition of caspule shell | NA | |||
| Pharmaceutical Development | To be updated soon | |||
| Manufacture of the product | To be updated soon | |||
| Tablet / Capsule Image | ||||
| Appearance | White to light yellow, oval shaped debossed with “3” on one side and “novo” on the other side | White to light yellow, oval shaped debossed with “7” on one side and “novo” on the other side | White to light yellow, oval shaped debossed with “14” on one side and “novo” on the other side | |
| Imprint code / Engraving / Debossment | Debossed with “3” on one side and “novo” on the other side | Debossed with “7” on one side and “novo” on the other side | Debossed with “14” on one side and “novo” on the other side | |
| Score | No score | No score | No score | |
| Color | White to light yellow | White to light yellow | White to light yellow | |
| Shape | Oval | Oval | Oval | |
| Dimension | 14mm | 14mm | 14mm | |
| Mfg by |
Novo Nordisk A/S DK-2880 Bagsvaerd Denmark |
|||
| Mfg for | - | |||
| Marketed by | - | |||
| Distributed by | - | |||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N213051 | 1,2,3 | 8129343 | January 29,2029 | DS | DP | U-2628 | - | Download |
| N213051 | 1,2,3 | 8536122 | March 20,2026 | DS | DP | U-2628 | - | Download |
| N213051 | 1,2,3 | 9278123 | December 16,2031 | - | DP | U-2628 | - | Download |
| N213051 | 1,2,3 | 10086047 | December 16,2031 | - | DP | - | - | Download |
| N213051 | 1,2,3 | 10278923 | February 5, 2034 | - | - | U-2628 | - | Download |
| N213051 | 1,2,3 | 10933120 | March 15,2033 | - | DP | - | - | |
| N213051 | 1,2,3 | 10960052 | December 16,2031 | - | DP | - | - |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| USP apparatus II | 70 rpm | 50 mM phosphate buffer (pH 6.8) with 0.05% Brij 35 | 500 mL | Q % in 45 minutes | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
