| Active Ingredient | SARECYCLINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEYSARA | 209521 | ALLERGAN INC | TABLET;ORAL | 60 MG, 100 MG and 150 MG | 150 MG | October 1, 2018 | October 1, 2023 | _ | Type 1 - New Molecular Entity | STANDARD | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
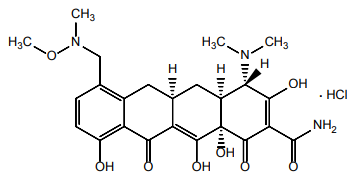 |
| Chemical Name | (4S,4aS,5aR,12aS)-4-(dimethylamino)-3,10,12,12a-tetrahydroxy-7-[(methoxy- (methyl)-amino)- methyl]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide monohydrochloride |
| CAS No | 1035979-44-2 |
| Molecular Formula | C24H29N3O8.HCl |
| Molecular Weight | 523.96 |
| Appearance | Yellow to slightly green powder |
| Solubility | It is methanol, slightly soluble in ethanol, and practically insoluble in acetonitrile. Its aqueous solubility increases with increasing pH (226 mg/mL at pH 8.0). It has pKa values of 2.03, 3.30, 7.60 and 9.92 |
| Water Solubility | It is sparingly soluble in water |
| Polymorphism | - |
| pKa (Strongest Acidic) | 2.95 (For Sarecycline) |
| pKa (Strongest Basic) | 8.26 (For Sarecycline) |
| Log P | -0.17 (For Sarecycline) |
| Identification | - |
| Degradation | - |
| Hygroscopic | Slightly hygroscopic |
| Photostability study | Sensitive to light |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | SEYSARA™ is a tetracycline-class drug indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older. Limitations of Use Efficacy of SEYSARA beyond 12 weeks and safety beyond 12 months have not been established. SEYSARA has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, SEYSARA should be used only as indicated |
| Dosage and Administration |
The recommended dosage of SEYSARA is once daily with or without food: • 60 mg for patients who weigh 33-54 kg, • 100 mg for patients who weigh 55-84 kg, • 150 mg for patients who weigh 85-136 kg. |
| Mechanism of action | The mechanism of action of SEYSARA in treating acne vulgaris is not known. |
| Absorption |
Increasing the SEYSARA dose from 60 to 150 mg once daily in healthy subjects resulted in a slightly less than proportional increase in sarcyeline steady-state Cmax and AUCtau. A mean accumulation ratio of sarecycline ranges from 1.5 to 1.6 fold with repeated dosing. Steady-state of sarecycline was reached by Day 7. Absorption The median time to peak plasma concentration (Tmax) of sarecycline is 1.5 to 2.0 hours. |
| Food Effect | Coadministration with a high-fat (approximately 50% of total caloric content of the meal), high-calorie (800 to 1000 Kcal) meal that included milk delayed Tmax by approximately 0.53 hour and decreased sarecycline Cmax by 31% and AUC by 27%. |
| Distribution | Protein binding of sarecycline is 62.5% to 74.7% in vitro. The mean apparent volume of distribution of sarecycline at steady-state ranges from 91.4 L to 97.0 L. |
| Metabolism | Metabolism of sarecycline by enzymes in human liver microsomes is minimal (< 15%) in vitro. Minor metabolites resulting from non-enzymic epimerization, O-/N-demethylation, hydroxylation, and desaturation have been found |
| Elimination |
The mean apparent oral clearance (CL/F) of sarecycline at steady state is approximately 3 L/h. The mean elimination half-life of sarecycline is 21 to 22 hours. Excretion After a single 100 mg oral dose of radiolabeled sarecycline, 42.6% of the dose was recovered in feces (14.9% as unchanged) and 44.1% in urine (24.7% as unchanged). |
| Peak plasma time (Tmax) | 1.5 to 2.0 hours |
| Half life | 21 to 22 hours |
| Bioavailability | - |
| Age, gender | No clinically significant differences in the pharmacokinetics of sarecycline were observed based on age (11 to 73 years), weight (42 to 133 kg), sex, renal impairment, or mild to moderate hepatic impairment (Child Pugh A to B). The effect of end-stage renal disease (ESRD) or severe hepatic impairment (Child-Pugh C) on sarecycline pharmacokinetics has not been assessed. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 60 MG | 100 MG | 150 MG | |
| Excipients used | Microcrystalline cellulose, povidone, sodium starch glycolate, and sodium stearyl fumarate | |||
| Composition of coating material | D&C yellow #10 aluminium lake, iron oxide yellow, methacrylic acid copolymer type C, polyethylene glycol, polyvinyl alcohol, sodium bicarbonate, talc, and titatnium dioxide | |||
| Composition of caspule shell | NA | |||
| Pharmaceutical Development | SEYSARA tablets contain 64.5 mg, 107.5 mg, and 161.2 mg of sarecycline hydrochloride equivalent to 60 mg,100 mg, and 150 mg sarecycline respectively | |||
| Manufacture of the product | Updated soon | |||
| Tablet / Capsule Image | ||||
| Appearance | Capsule-shaped, yellow, film-coated tablets debossed with“S60” on one side and blank on the other side. | Capsule-shaped, yellow, film-coated tablets debossed with “S100” on one side and blank on the other side | Capsule-shaped, yellow, film-coated tablets debossed with “S150” on one side and blank on the other side. | |
| Imprint code / Engraving / Debossment | Debossed with“S60” on one side and blank on the other side. | Debossed with“S100” on one side and blank on the other side. | Debossed with“S150” on one side and blank on the other side. | |
| Score | No score | No score | No score | |
| Color | Yellow | Yellow | Yellow | |
| Shape | Capsule | Capsule | Capsule | |
| Dimension | 13 mm | 15 mm | 17 mm | |
| Mfg by | - | |||
| Mfg for | - | |||
| Marketed by | - | |||
| Distributed by |
Allergan USA, Inc. Madison, NJ 07940 USA |
|||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N209521 | 1 | 8318706 | May 1, 2031 | DS | DP | U-2405 | - | Download |
| N209521 | 1 | 8513223 | December 7, 2029 | - | - | U-2406 | - | Download |
| N209521 | 1 | 9255068 | February 9, 2033 | DS | DP | U-2407, U-2408 | - | Download |
| N209521 | 1 | 9481639 | August 10, 2028 | - | - | U-2409 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| USP Apparatus 2 - Paddle | 75 | 0.1 N HCl | 500 | Q point in 15 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
