| Active Ingredient | SACUBITRIL; VALSARTAN |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENTRESTO | (NDA) 207620 | NOVARTIS PHARMS CORP | TABLET;ORAL | 24MG; 26MG 49MG; 51MG 97MG; 103MG | 97MG; 103MG (RS) | July 7, 2015 | July 7, 2020 | - | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
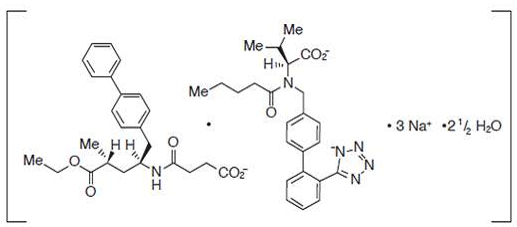 |
| Chemical Name | ENTRESTO contains a co-crystal complex comprised of anionic forms of sacubitril and valsartan,sodium cations, and water molecules in the molar ratio of 1:1:3:2.5, respectively. Following oral administration, the complex dissociates into sacubitril (which is further metabolized to LBQ657) and valsartan. The complex is chemically described as Octadecasodiumhexakis(4-{[(1S,3R)-1-([1,1´-biphenyl]-4-ylmethyl)-4-ethoxy-3-methyl-4-oxobutyl]amino}-4oxobutanoate)hexakis(N-pentanoyl-N-{[2´-(1H-tetrazol-1-id-5-yl)[1,1´-biphenyl]-4-yl]methyl}-L-valinate)—water. (complex dissociates on dissolution which allows the active moieties to act independently in vivo.) |
| CAS No | (1) Sacubitril: 149709-62-6 (2) Valsartan: 137862-53-4 |
| Molecular Formula | C48H55N6O8Na32.5 H2O (hemipentahydrate) |
| Molecular Weight | 957.99 (hemipentahydrate) |
| Appearance | a white to almost white crystalline powder |
| Solubility | Both active substances exhibit pH dependent solubility in aqueous media, being freely soluble at neutral pH but much less soluble at lower pH. Both are soluble in alcoholic solvents but less so in aprotic organic media. |
| Water Solubility | Valsartan: 0.0234 mg/mL (Predicted) |
| Polymorphism | Polymorphism has not been observed for the co-crystal complex. The sacubitril-valsartan complex was shown to be stable with respect to changes in polymorphic form in several studies. |
| pKa (Strongest Acidic) | Valsartan: 4.37 (Predicted) |
| pKa (Strongest Basic) | Valsartan: -0.11 (Predicted) |
| Log P | Valsartan:5.8 |
| Identification | IR, UV, XRPD |
| Degradation | The co-crystal complex deliquesces at high humidity, more rapidly at increased temperature, along with a decrease in assay and increase in impurities. Sacubitril degrades under acidic and basic conditions whilst valsartan is susceptible to oxidative degradation. |
| Hygroscopic | hygroscopic above 60% RH |
| Photostability study | not photosensitive |
| Melting Point | Valsartan: 116-117 °C |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | ENTRESTO is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. ENTRESTO is usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB. |
| Dosage and Administration | ENTRESTO is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to ENTRESTO allow a washout periodof 36 hours between administration of the two drugs. The recommended starting dose of ENTRESTO is 49/51 mg twice-daily. Double the dose of ENTRESTO after 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient. |
| Mechanism of action |
Sacubitril is a pro-drug, in vivo ester hydrolysis affording the carboxylic acid which is responsible for the therapeutic effect. Both sacubitril and its metabolite can be considered new active substances since neither is structurally related, (as salts, esters, ethers, isomers, mixtures of isomers, complexes or derivatives), to any other active substance. ENTRESTO contains a neprilysin inhibitor, sacubitril, and an angiotensin receptor blocker, valsartan. ENTRESTO inhibits neprilysin (neutral endopeptidase; NEP) via LBQ657,the active metabolite of the prodrug sacubitril, and blocks the angiotensin II type-1 (AT1) receptor via valsartan. The cardiovascular and renal effects of ENTRESTO in heart failure patients are attributed to the increased levels of peptides thatare degraded by neprilysin, such as natriuretic peptides, by LBQ657, and the simultaneous inhibition of the effects of angiotensin II by valsartan. Valsartan inhibits the effects of angiotensin II by selectively blocking the AT1receptor, and also inhibits angiotensin II-dependent aldosterone release. |
| Absorption |
The pharmacokinetics of sacubitril, LBQ657, and valsartan were linear over an ENTRESTO dose range of 24 mg sacubitril/26 mg valsartan to 194 mg sacubitril/206 mg valsartan. Following oral administration, ENTRESTO dissociates into sacubitril and valsartan. Sacubitril is further metabolized to LBQ657. The peak plasma concentrations of sacubitril, LBQ657,and valsartan are reached in0.5 hours, 2 hours, and 1.5 hours, respectively. The oral absolute bioavailability of sacubitril is estimated to be ≥60%. The valsartan in ENTRESTO is more bioavailable than the valsartan in other marketed tablet formulations; 26 mg, 51 mg, and 103 mg of valsartan in ENTRESTO is equivalent to 40 mg, 80 mg, and 160 mg of valsartan in other marketed tablet formulations, respectively. Following twice-daily dosing of ENTRESTO, steady state levelsof sacubitril, LBQ657, and valsartan are reached in 3 days. At steady state, sacubitril and valsartan do not accumulate significantly, whereas LBQ657 accumulates by 1.6-fold. |
| Food Effect | ENTRESTO administration with food has no clinically significant effect on the systemic exposures of sacubitril, LBQ657, or valsartan. Although there is a decrease in exposure to valsartan when ENTRESTO is administered with food, this decrease is not accompanied by a clinically significant reduction in the therapeutic effect. ENTRESTO can therefore be administered with or without food. |
| Distribution | Sacubitril, LBQ657 and valsartan are highly bound to plasma proteins (94% to 97%). Based on the comparison of plasma and CSF exposures, LBQ657 crosses the blood brain barrier to a limited extent (0.28%). The average apparent volumes of distribution of valsartan and sacubitril are 75 and 103 L, respectively. |
| Metabolism | Sacubitril is readily converted to LBQ657 by esterases; LBQ657 is not further metabolized to a significant extent. Valsartan is minimally metabolized; only about 20% of the dose is recovered as metabolites. A hydroxyl metabolite has been identified in plasma at low concentrations (< 10%). |
| Elimination | Following oral administration, 52% to 68% of sacubitril (primarily as LBQ657) and ~13% of valsartan and its metabolites are excreted in urine; 37% to 48% ofsacubitril (primarily as LBQ657), and 86%of valsartan and its metabolites are excreted in feces. Sacubitril, LBQ657, and valsartan are eliminated from plasma with a mean elimination half-life (T1/2) of approximately 1.4 hours, 11.5 hours, and 9.9 hours, respectively. |
| Peak plasma time (Tmax) | 0.5 hours (sacubitril), 2 hours (LBQ657), and 1.5 hours (valsartan) |
| Half life | 1.4 hours (sacubitril), 11.5 hours (LBQ657), and 9.9 hours (valsartan) |
| Bioavailability | ≥60% (sacubitril) |
| Age, gender | No relevant pharmacokinetic differenceshave been observed in elderly (≥65 years) or very elderly (≥75 years) patients compared to the overall population. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 17897 | A | II | December 15, 2004 | SUN PHARMACEUTICAL INDUSTRIES LTD (VALSARTAN USP) |
| 17909 | A | II | December 16, 2004 | TEVA PHARMACEUTICAL INDUSTRIES LTD (VALSARTAN) |
| 18253 | A | II | April 7, 2005 | MYLAN LABORATORIES LTD (VALSARTAN USP) |
| 18967 | A | II | November 22, 2005 | JUBILANT GENERICS LTD (VALSARTAN USP) |
| 19301 | A | II | March 27, 2006 | LUPIN LTD (VALSARTAN USP) |
| 20319 | A | II | March 2, 2007 | DR REDDYS LABORATORIES LTD (VALSARTAN) |
| 20939 | A | II | September 24, 2007 | ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD (VALSARTAN DRUG SUBSTANCE) |
| 21134 | A | II | November 15, 2007 | ALEMBIC PHARMACEUTICALS LTD [Valsartan USP ( PROCESS I)] |
| 21845 | A | II | July 30, 2008 | AUROBINDO PHARMA LTD [VALSARTAN USP (NON-STERILE DRUG SUBSTANCE)] |
| 22183 | I | II | November 14, 2008 | CADILA PHARMACEUTICALS LTD (VALSARTAN) |
| 22614 | I | II | April 1, 2010 | IPCA LABORATORIES LTD (VALSARTAN USP) |
| 22684 | A | II | March 31, 2009 | CADILA HEALTHCARE LTD (VALSARTAN USP) |
| 23150 | A | II | October 5, 2009 | SIGNA SA DE CV VALSARTAN USP |
| 23491 | A | II | January 22, 2010 | ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD [VALSARTAN USP (PROCESS II)] |
| 23584 | A | II | February 24, 2010 | ALEMBIC PHARMACEUTICALS LTD [VALSARTAN USP (PROCESS II)] |
| 23902 | A | II | July 2, 2010 | NOVARTIS PHARMACEUTICALS CORP (VALSARTAN DRUG SUBSTANCE) |
| 24178 | A | II | September 13, 2010 | MACLEODS PHARMACEUTICALS LTD (VALSARTAN) |
| 24544 | A | II | March 2, 2011 | AUROBINDO PHARMA LTD [VALSARTAN USP (PROCESS II)] |
| 24724 | A | II | January 18, 2011 | TEVA PHARMACEUTICAL INDUSTRIES LTD (VALSARTAN) |
| 24797 | A | II | March 29, 2011 | DIVIS LABORATORIES LTD (VALSARTAN USP) |
| 24873 | A | II | April 18, 2011 | HETERO LABS LTD (VALSARTAN) |
| 25510 | I | II | November 14, 2011 | ZHUHAI RUNDU PHARMACEUTICAL CO LTD (VALSARTAN) |
| 25848 | A | II | March 1, 2012 | SECOND PHARMA CO LTD (VALSARTAN) |
| 26167 | A | II | July 7, 2012 | CADILA PHARMACEUTICALS LTD [VALSARTAN USP (PROCESS 2)] |
| 26397 | A | II | September 6, 2012 | HETERO LABS LTD [VALSARTAN (PROCESS II)] |
| 26690 | A | II | December 19, 2012 | ZHEJIANG HISUN PHARMACEUTICAL CO LTD (VALSARTAN) |
| 27780 | A | II | December 27, 2013 | ALEMBIC PHARMACEUTICALS LTD [VALSARTAN USP (PROCESS III)] |
| 29392 | A | II | May 29, 2015 | HETERO LABS LTD [VALSARTAN (PROCESS-III)] |
| 31271 | A | II | December 30, 2016 | MYLAN LABORATORIES LTD (SACUBITRIL) |
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 24+26 MG | 49+51 MG | 97+103 MG | |
| Excipients used | microcrystalline cellulose, low-substituted hydroxypropylcellulose, crospovidone, magnesium stearate (vegetable origin), talc, and colloidalsilicon dioxide | |||
| Composition of coating material | hypromellose, titanium dioxide (E171), Macrogol 4000,talc, iron oxide red (E172) and iron oxide black (E172) | hypromellose, titanium dioxide (E171), Macrogol 4000, talc, iron oxide red (E172) and iron oxide yellow (E172) |
hypromellose, titanium dioxide (E171), Macrogol 4000,talc, iron oxide red (E172) and iron oxide black (E172) |
|
| Composition of caspule shell | - | |||
| Pharmaceutical Development |
The aim of development was to identify an immediate release solid oral dosage form of sacubitril and valsartan. The co-crystal form identified by the applicant renders valsartan more bioavailable than in its standalone formulations and the dose was reduced accordingly. Both active substances are highly soluble in aqueous media above pH 5 and show medium permeability. The dry-granulation roller compaction process was used to supply later trials and the commercial market. Additionally, a film-coating step was introduced, both for taste masking purposes and to easily distinguish between the different strengths based on their colour. In order to develop a robust roller compaction step, multivariate experiments were carried out optimising compression force, screen size and the amounts of talc and magnesium stearate. The middle and high strength tablets are dose proportional. A clinical bioequivalence study was carried out on lower strength as it has no dose proportional formula. |
|||
| Manufacture of the product |
The manufacturing process consists of six main steps: blending of intra-granular excipients; roller compaction; blending with extra-granular excipients; compression; film-coating; packaging. The roller compaction force is critical to tablet hardness and subsequent dissolution and limits have been set accordingly. |
|||
| Tablet / Capsule Image |
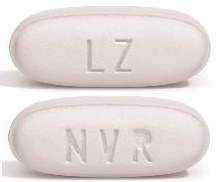
|
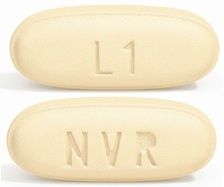
|
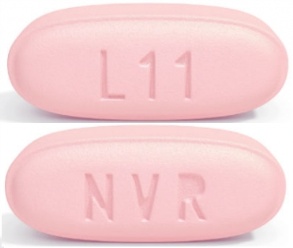
|
|
| Appearance | ovaloid, biconvex, violet white film-coated tablets and debossed with “NVR” on one side and “LZ” on the other side | ovaloid, biconvex, pale yellow film-coated tablets and debossed with “NVR” on one side and “L1” on the other side | ovaloid, biconvex, light pink film-coated tablets and debossed with “NVR” on one side and “L11” on the other side | |
| Imprint code / Engraving / Debossment | debossed with “NVR” on one side and “LZ” on the other side | debossed with “NVR” on one side and “L1” on the other side | debossed with “NVR” on one side and “L11” on the other side | |
| Score | no score | no score | no score | |
| Color | VIOLET WHITE | PALE YELLOW | LIGHT PINK | |
| Shape | OVAL (Ovaloid Biconvex) | OVAL (Ovaloid Biconvex) | OVAL (Ovaloid Biconvex) | |
| Dimension | 13mm × 5.5mm | 13mm × 5.5mm | 15mm × 6mm | |
| Mfg by | Novartis Pharmaceuticals Corporation (US, EU) | |||
| Mfg for | - | |||
| Marketed by | Novartis Pharmaceuticals Corporation (US, EU) | |||
| Distributed by | Novartis Pharmaceuticals Corporation (US, EU) | |||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N207620 | 1 | 7468390 | November 27, 2023 | - | Y | - | - | Download |
| N207620 | 1 | 8796331 | January 14, 2023 | - | - | U - 1723 | - | Download |
| N207620 | 1 | 8877938 | May 27, 2027 | Y | Y | - | - | Download |
| N207620 | 1 | 8101659 | January 14, 2023 | - | DP | - | - | Download |
| N207620 | 1 | 8404744 | January 14, 2023 | - | DP | - | - | Download |
| N207620 | 1 | 9388134 | November 8, 2026 | - | - | U-1723 | - | Download |
| - | - | - | - | - | - | - |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | Phosphate Buffer, pH 6.8[degassed] | 900 | 10, 15, 20, 30 and 45 | March 17, 2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | ENTRESTO | Download |
| UK | ENTRESTO | Download |
| US | ENTRESTO | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
