| Active Ingredient | RELUGOLIX |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORGOVYX | 214621 | MYOVANT SCIENCES | TABLET;ORAL | 120MG | 120MG | December 18, 2020 | December 18, 2025 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
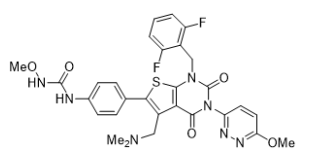 |
| Chemical Name | N-(4-{1[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N’-methoxyurea. |
| CAS No | 737789-87-6 |
| Molecular Formula | C29H27F2N7O5S |
| Molecular Weight | 623.63 daltons |
| Appearance | White to off-white to slightly yellow solid |
| Solubility | Solubility of 0.04 mg per mL in water at 25°C. |
| Water Solubility | 0.00198 mg/mL |
| Polymorphism | - |
| pKa (Strongest Acidic) | 9.07 |
| pKa (Strongest Basic) | 7.69 |
| Log P | 3.16, 3.94 |
| Identification | UV and HPLC retention times |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | BCS Class IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | ORGOVYX is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer. |
| Dosage and Administration | Recommended Dosage: A loading dose of 360 mg on the first day of treatment followed by 120 mg taken orally once daily, at approximately the same time each day. ORGOVYX can be taken with or without food. Instruct patients to swallow tablets whole and not to crush or chew tablets |
| Mechanism of action | Relugolix is a nonpeptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby, reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and consequently testosterone |
| Absorption | Relugolix is a substrate for intestinal P-gp. The mean (CV%) absolute bioavailability of relugolix is approximately 12% (62%). The median (range) Tmax of relugolix is 2.25 hours (0.5 to 5.0 hours). |
| Food Effect | No clinically meaningful differences in the pharmacokinetics of relugolix were observed following consumption of a high-calorie, high-fat meal (approximately 800 to 1000 calories with 500, 220, and 124 from fat, carbohydrate, and protein, respectively). |
| Distribution | Plasma protein binding of relugolix is 68 to 71%, primarily to albumin and to a lesser extent to α1-acid glycoprotein. The mean blood-to-plasma ratio is 0.78. |
| Metabolism | Relugolix is metabolized primarily by CYP3A and to a lesser extent by CYP2C8 in vitro. |
| Elimination |
Elimination : The mean effective half-life of relugolix is 25 hours and the mean (CV%) terminal elimination half-life is 60.8 (11%) hours. The mean (CV%) total clearance of relugolix is 29.4 (15%) L/h and the renal clearance is 8 L/h. Excretion : After oral administration of a single 80-mg radiolabeled dose of relugolix, approximately 81% of the radioactivity was recovered in feces (4.2% as unchanged) and 4.1% in urine (2.2% as unchanged). |
| Peak plasma time (Tmax) | 2.25 hours (0.5 to 5.0 hours) |
| Half life | The mean effective half-life of relugolix is 25 hours and the mean (CV%) terminal elimination half-life is 60.8 (11%) hours |
| Bioavailability | 12% (62%) |
| Age, gender | No clinically meaningful differences in the pharmacokinetics of relugolix were observed based on age (45 to 91 years), race/ethnicity (Asian [19%], White [71%], Black/African American [6%]), body weight (41 to 193 kg), mild to severe renal impairment (creatinine clearance [CLcr] 15 to 89 mL/min, as estimated by the Cockcroft-Gault equation), or mild to moderate hepatic impairment (Child-Pugh A or B). The effect of end-stage renal disease with or without hemodialysis or severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of relugolix has not been evaluated. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 120 MG |
| Excipients used | Mannitol, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate |
| Composition of coating material | Hypromellose, titanium dioxide, ferric oxide red, and carnauba wax |
| Composition of caspule shell | NA |
| Pharmaceutical Development | NA |
| Manufacture of the product | NA |
| Tablet / Capsule Image |

|
| Appearance | Film-coated, light red, almond shaped, and debossed with “R” on one side and “120” on the other side |
| Imprint code / Engraving / Debossment | Debossed with “R” on one side and “120” on the other side |
| Score | No score |
| Color | RED (light red) |
| Shape | FREEFORM (almond) |
| Dimension | 11mm |
| Mfg by | - |
| Mfg for | Myovant Sciences, Inc., Brisbane, CA 94005 |
| Marketed by | Bushu Pharmaceuticals, Ltd, Kawagoe, Saitama, Japan |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N214621 | 1 | 7300935 | January 28, 2024 | DS | - | - | - | Download |
| N214621 | 1 | 8058280 | January 28, 2024 | DS | DP | - | - | Download |
| N214621 | 1 | 8735401 | April 2, 2024 | - | - | U-3019 | - | Download |
| N214621 | 1 | 10350170 | February 25, 2036 | - | DP | - | - | Download |
| N214621 | 1 | 10449191 | September 29, 2037 | - | - | U-3020 | - | |
| N214621 | 1 | 10786501 | September 29, 2037 | - | - | U-3020 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| USP Apparatus 2 (paddles) | 50rpm | 50 mM citrate buffer, pH 5.5 | 900 mL | 0, 15, 30, 45 minutes | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
