| Active Ingredient | REGORAFENIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STIVARGA | (NDA) 203085 | BAYER HLTHCARE | TABLET;ORAL | 40MG | 40MG (RS) | September 27, 2012 | September 27, 2017 | Feb 25, 2020 Apr 27, 2024 | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
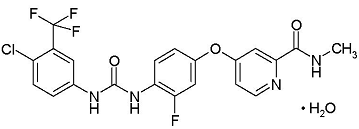 |
| Chemical Name | 4-[4-({[4-chloro-3-(trifluoromethyl) phenyl] carbamoyl} amino)-3fluorophenoxy]-N-methylpyridine-2-carboxamide monohydrate |
| CAS No | 755037-03-7 |
| Molecular Formula | C21H15ClF4N4O3• H2O |
| Molecular Weight | 500.83 |
| Appearance | a white to slightly pink or slightly brownish solid substance |
| Solubility | practically insoluble in water, dilute alkaline solution, dilute acid solution, n-heptane, glycerine and toluene. It is slightly soluble in acetonitrile, dichloromethane, propylene glycol, methanol, 2-propanol, ethanol and ethyl acetate. It is sparingly soluble in acetone and soluble in PEG 400 (macrogol) |
| Water Solubility | 0.00102 mg/mL |
| Polymorphism | Regorafenib crystallizes in three modifications with melting points at 206 °C (Mod. I), at 181 °C (Mod. II,) and at 141 °C (Mod. III). In addition, one pseudo-polymorph has been found, a monohydrate (water content of 3.6 %). |
| pKa (Strongest Acidic) | 10.52 |
| pKa (Strongest Basic) | 2.02 |
| Log P | 4.53 |
| Identification | IR, HPLC |
| Degradation | Results on stress conditions (thermal, oxidative, and hydrolytic stress) show that regorafenib is chemically extremely stable to thermal stress, has good stability towards hydrolytic stress and is quite stable to oxidative stress. |
| Hygroscopic | not hygroscopic |
| Photostability study | photo stable |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | Regorafenib is synthesized in three main steps using well defined starting materials with acceptable specification. Adequate in-process controls are applied during the synthesis. The specifications and control methods for intermediate products, starting materials and reagents have been presented. The active substance is packed in polypropylene or polyethylene bags. |
| Parameters | Details |
|---|---|
| Indications and Usage | Colorectal Cancer : Stivarga is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy. Gastrointestinal Stromal Tumors: Stivarga is indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumor (GIST) who have been previously treated with imatinib mesylate and sunitinib malate. |
| Dosage and Administration | The recommended dose is 160 mg regorafenib (four 40 mg tablets) taken orally once daily for the first 21 days of each 28day cycle. Continue treatment until disease progression or unacceptable toxicity. Take Stivarga at the same time each day. Swallow tablet whole with water after a low-fat meal that contains less than 600 calories and less than 30% fat. Do not take two doses of Stivarga on the same day to make up for a missed dose from the previous day. |
| Mechanism of action | Regorafenib is a small molecule inhibitor of multiple membrane-bound and intracellular kinases involved in normal cellular functions and in pathologic processes such as oncogenesis, tumor angiogenesis, and maintenance of the tumor microenvironment. In in vitrobiochemical or cellular assays, regorafenib or its major human active metabolites M-2 and M-5 inhibited the activity of RET, VEGFR1, VEGFR2, VEGFR3, KIT, PDGFR-alpha, PDGFR-beta, FGFR1, FGFR2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF, BRAF V600E, SAPK2, PTK5, and Abl at concentrations of regorafenib that have been achieved clinically. In in vivo models, regorafenib demonstrated anti-angiogenic activity in a rat tumor model, and inhibition of tumor growth as well as anti-metastatic activity in several mouse xenograft models including some for human colorectal carcinoma. |
| Absorption | Following a single 160 mg dose of Stivarga in patients with advanced solid tumors, regorafenib reaches a geometric mean peak plasma level (Cmax) of 2.5 µg/mL at a median time of 4 hours and a geometric mean area under the plasma concentration vs. time curve (AUC) of 70.4 µg*h/mL. The AUC of regorafenib at steady-state increases less than dose proportionally at doses greater than 60 mg. At steady-state, regorafenib reaches a geometric mean Cmaxof 3.9 µg/mL and a geometric mean AUC of 58.3 µg*h/mL. The coefficient of variation of AUC and Cmax is between 35% and 44%. The mean relative bioavailability of tablets compared to an oral solution is 69% to 83%. |
| Food Effect | In a food-effect study, 24 healthy men received a single 160 mg dose of Stivarga on three separate occasions: under a fasted state, with a high-fat meal and with a low-fat meal. A high-fat meal (945 calories and 54.6 g fat) increased the mean AUC of regorafenib by 48% and decreased the mean AUC of the M-2 and M-5 metabolites by 20% and 51%, respectively, as compared to the fasted state. A low-fat meal (319 calories and 8.2 g fat) increased the mean AUC of regorafenib, M-2 and M-5 by 36%, 40% and 23%, respectively as compared to fasted conditions. Stivarga was administered with a low-fat meal in Studies 1 and 2. |
| Distribution | Regorafenib undergoes enterohepatic circulation with multiple plasma concentration peaks observed across the 24-hour dosing interval. Regorafenib is highly bound (99.5%) to human plasma proteins. |
| Metabolism | Regorafenib is metabolized by CYP3A4 and UGT1A9. The main circulating metabolites of regorafenib measured at steady-state in human plasma are M-2 (N-oxide) and M-5 (N-oxide and N-desmethyl), both of them having similar in vitropharmacological activity and steady-state concentrations as regorafenib. M-2 and M-5 are highly protein bound (99.8% and 99.95%, respectively). |
| Elimination |
Following a single 160 mg oral dose of Stivarga, the geometric mean (range) elimination half-lives for regorafenib and the M-2 metabolite in plasma are 28 hours (14 to 58 hours) and 25 hours (14 to 32 hours), respectively. M-5 has a longer mean (range) elimination half-life of 51 hours (32 to 70 hours). Approximately 71% of a radiolabeled dose was excreted infeces (47% as parent compound, 24% as metabolites) and 19% of the dose was excreted in urine (17% as glucuronides) within 12 days after administration of a radiolabeled oral solution at a dose of 120 mg. |
| Peak plasma time (Tmax) | 4 hours |
| Half life | 28 hours (14 to 58 hours of API) and 25 hours (14 to 32 hours of M-2 metabolite |
| Bioavailability | 69% to 83% |
| Age, gender | Based on the population pharmacokinetic analysis, there is no clinically relevant effect of age, gender or weight on the pharmacokinetics of regorafenib. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 29870 | A | II | November 3, 2015 | MSN LABORATORIES PRIVATE LTD |
| 30490 | A | II | May 13, 2016 | HETERO LABS LTD |
| Parameters | Details |
|---|---|
| Strength | 40MG |
| Excipients used | cellulose microcrystalline (100MG), croscarmellose sodium (154MG), magnesium stearate (3.6MG), povidone (160MG), and colloidal silicon dioxide (2.4MG) |
| Composition of coating material | ferric oxide red, ferric oxide yellow and titanium dioxide, lecithin, polyethylene glycol 3350, polyvinyl alcohol and talc. |
| Composition of caspule shell | - |
| Pharmaceutical Development |
The objective of the pharmaceutical development was to provide an immediate release solid dosage form of regorafenib with high oral bioavailability and high patient compliance. A solid solution (co-precipitate) tablet formulation was selected as dosage form, in order to transfer the active substance, which is characterized by an extremely low solubility in aqueous media, into the amorphous form. Upon contact with the dissolution medium the tablets disintegrate and the solid solution dissolves forming a supersaturated solution with a significantly higher concentration of regorafenib in solution than expected based on the solubility of the crystalline active substance. Consequently, higher oral bioavailability is achieved when administering regorafenib as a solid solution tablet compared to a conventional tablet comprising the active substance in crystalline micronized form. Regorafenib tablets are film-coated in order to provide a homogeneous appearance, to add a colour for product identification, to reduce dusting during handling of the tablets and to facilitate swallowing. The dissolution method has been adequately developed and its discriminating capability demonstrated. The use of surfactant and the dissolution medium was justified. Sink conditions were confirmed. The dissolution test was shown to be sufficiently discriminative in detecting relevant changes to the solid solution granules. The finished product is sensitive to moisture. 40MG of regorafenib anhydrous is equivalent to 41.49MG of regorafenib monohydrate. |
| Manufacture of the product | The manufacture of the finished product involves conventional processes including (1) mixing, (2) granulation, (3) roller compaction, (4) blending, (5) post-blending, (6) tableting, (7) coating and (8) drying |
| Tablet / Capsule Image |
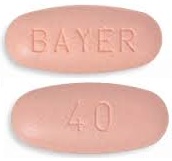
|
| Appearance | light pink, oval shaped, film-coated tablet, debossed with ‘BAYER’ on one side and ‘40’ on the other side. |
| Imprint code / Engraving / Debossment | debossed with ‘BAYER’ on one side and ‘40’ on the other side. |
| Score | no score |
| Color | LIGHT PINK |
| Shape | OVAL |
| Dimension | 16mm |
| Mfg by | Bayer HealthCare (EU) |
| Mfg for | Bayer HealthCare (US) |
| Marketed by | Bayer HealthCare (EU) |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N203085 | 1 | 7351834 | January 28, 2022 | Y | - | - | - | Download |
| N203085 | 1 | 8637553 | February 16, 2031 | Y | Y | - | - | Download |
| N203085 | 1 | 8680124 | June 2, 2030 | - | - | U - 1506 | - | Download |
| N203085 | 1 | 9458107 | April 8, 2031 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | Acetate Buffer pH 4.5 with 0.1% Sodium Dodecyl Sulfate (SDS) | 900 | 10, 15, 20, 30 and 45 | June 25, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | STIVARGA | Download |
| UK | STIVARGA | Download |
| US | STIVARGA | Download |
| Exclusivity Code: Exclusivity Expiration is I -744: Apr 27, 2020 |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
