| Active Ingredient | RAMELTEON |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROZEREM | (NDA) 021782 | TAKEDA PHARMS USA | TABLET;ORAL | 8 MG | 8 MG | July 22, 2005 | _ | _ | 1 New molecular entity (NME) | S Standard review drug | Prescription | AB |
| Parameters | Details |
|---|---|
| Structural Formula |
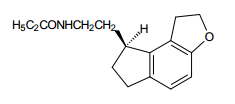 |
| Chemical Name | (S)-N-[2-(1, 6, 7, 8tetrahydro-2H-indeno-[5, 4-b]furan-8-yl)ethyl]propionamide |
| CAS No | 196597-26-9 |
| Molecular Formula | C16H21NO2 |
| Molecular Weight | 259.34 |
| Appearance | - |
| Solubility | Freely soluble in organic solvents, such asmethanol, ethanol, and dimethyl sulfoxide; soluble in 1-octanol and acetonitrile; and veryslightly soluble in aqueous buffers from pH 3 to pH 11 |
| Water Solubility | Veryslightly soluble in water |
| Polymorphism | - |
| pKa (Strongest Acidic) | 15.82 (Predicted) |
| pKa (Strongest Basic) | -0.4 (Predicted) |
| Log P | 2.4 |
| Identification | - |
| Degradation | Some degradation products have been encountered in aqueous solution |
| Hygroscopic | Not hygroscopic |
| Photostability study | Light labile |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | The synthesis is a 4-step process. Starting materials are characterised and controlled to maintain the purity of the resulting active substance. The chiral centre is introduced during synthesis and process investigations demonstrate the satisfactory retention of this centre resulting in the S-isomer. The active substance is purified by recrystallisation and micronised. The molecular structure has been confirmed by a wide range of spectroscopic methods, and there is no evidence of polymorphism. |
| Parameters | Details |
|---|---|
| Indications and Usage | ROZEREM is indicated for the treatment of insomnia characterized by difficulty with sleep onset |
| Dosage and Administration |
• Adult dose: 8 mg taken within 30 minutes of going to bed. • Should not be taken with or immediately after a high-fat meal. • Total daily dose should not exceed 8 mg. |
| Mechanism of action |
ROZEREM (ramelteon) is a melatonin receptor agonist with both high affinity for melatonin MT1and MT2 receptors and selectivity over the MT3receptor. Ramelteon demonstrates full agonist activity in vitroin cells expressing human MT1or MT2receptors. The activity of ramelteon at the MT1and MT2 receptors is believed to contribute to its sleep-promoting properties, as these receptors, acted upon by endogenous melatonin, are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle. Ramelteon has no appreciable affinity for the GABA receptor complex or for receptors that bind neuropeptides, cytokines, serotonin, dopamine, noradrenaline, acetylcholine, and opiates. Ramelteon also does not interfere with the activity of a number of selected enzymes in a standard panel. The major metabolite of ramelteon, M-II, is active and has approximately one tenth and one fifth the binding affinity of the parent molecule for the human MT1and MT2receptors, respectively, and is 17- to 25fold less potent than ramelteon in in vitrofunctional assays. Although the potency of M-II at MT1and MT2 receptors is lower than the parent drug, M-II circulatesat higher concentrations than the parent producing 20- to 100-fold greater mean systemic exposure when compared to ramelteon. M-II has weak affinity for the serotonin 5-HT2Breceptor, but no appreciable affinity for other receptors or enzymes. Similar to ramelteon, M-II does not interfere with the activity of a number of endogenous enzymes. All other known metabolites of ramelteon are inactive. |
| Absorption | Ramelteon is absorbed rapidly, with median peak concentrations occurring at approximately 0.75 hour (range, 0.5 to 1.5 hours) after fasted oral administration. Although the total absorption of ramelteon is at least 84%, the absolute oral bioavailability is only 1.8% due to extensive first-pass metabolism. |
| Food Effect | When administered witha high-fat meal, the AUC0-inffor a single 16 mg dose of ROZEREM was 31% higher and the Cmaxwas 22% lower than when given in a fasted state. Median Tmaxwas delayed by approximately 45 minutes when ROZEREM was administered with food. Effects of food on the AUC values for M-II were similar. It is therefore recommended that ROZEREM not be taken with or immediately after a high-fat meal. |
| Distribution | In vitroprotein binding of ramelteon is approximately 82% in human serum, independent of concentration. Binding to albumin accounts for most of that binding, since 70% of the drug is bound in human serum albumin. Ramelteon is not distributed selectively to red blood cells.Ramelteon has a mean volume of distribution after intravenous administration of 73.6 L, suggesting substantial tissue distribution. |
| Metabolism | Metabolism of ramelteon consists primarily of oxidation to hydroxyl and carbonyl derivatives, with secondary metabolism producing glucuronide conjugates. CYP1A2 is the major isozyme involved in the hepatic metabolism of ramelteon; the CYP2C subfamilyand CYP3A4 isozymes are also involved to a minor degree. The rank order of the principal metabolites by prevalence in human serum is M-II,M-IV, M-I, and M-III. These metabolites are formed rapidly and exhibit a monophasic decline and rapid elimination. The overall mean systemic exposure of M-II is approximately 20- to 100-fold higher than parent drug. |
| Elimination | Following oral administration of radiolabeled ramelteon, 84% of total radioactivity was excreted in urine and approximately 4% in feces, resulting in a mean recovery of 88%. Less than 0.1% of the dose was excreted in urine and feces as the parent compound. Elimination was essentially complete by 96 hours post-dose. Repeated once daily dosing with ROZEREM does not result in significant accumulation owing to the short elimination half-life of ramelteon (on average, approximately 1 to 2.6 hours). The half-life of M-II is 2 to 5 hours and independent of dose. Serum concentrations of the parent drug and its metabolites in humans are at or below the lower limits of quantitation within 24 hours. |
| Peak plasma time (Tmax) | 0.75 hour (range, 0.5 to 1.5 hours) after fasted oral administration. Tmaxwas delayed by approximately 45 minutes when ROZEREM was administered with food. |
| Half life | 2 to 5 hours |
| Bioavailability | 1.8% |
| Age, gender |
In a group of 24 elderly subjects aged 63 to 79 years administered a single ROZEREM 16 mg dose, the mean Cmaxand AUC0-infvalues were 11.6 ng/mL (SD, 13.8) and 18.7 ng·hr/mL (SD, 19.4), respectively. The elimination half-life was 2.6 hours (SD, 1.1). Compared with younger adults, the total exposure (AUC0-inf) and Cmaxof ramelteon were 97% and 86% higher, respectively, in elderly subjects. The AUC0-infand Cmaxof M-II were increased by 30% and 13%, respectively, in elderly subjects. There are no clinically meaningful gender-related differencesin the pharmacokinetics of ROZEREM or its metabolites. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 22882 | A | II | June 22, 2009 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| Parameters | Details |
|---|---|
| Strength | 8MG |
| Excipients used | Lactose monohydrate, starch, hydroxypropyl cellulose, magnesium stearate, hypromellose, copovidone |
| Composition of coating material | Titanium dioxide, yellow ferric oxide, polyethylene glycol 8000, and ink containing shellac and synthetic iron oxide black |
| Composition of caspule shell | - |
| Pharmaceutical Development | The main factor influencing development has been the limited aqueous solubility of the active substance, although considering the small dose, this is not a major problem. Following synthesis the particle size of the active substance is reduced, and the effect of particle size on dissolution has been studied. These studies allow a mean particle size to be defined, suitable for further processing in the manufacture of the product. Ramelteon is slightly sensitive to light and has a bitter taste. Therefore, film-coating was applied, using conventional excipients. |
| Manufacture of the product | The process is standard mixing, granulation, lubrication, film-coating process that has been well validated with regard to critical sub-processes. |
| Tablet / Capsule Image |
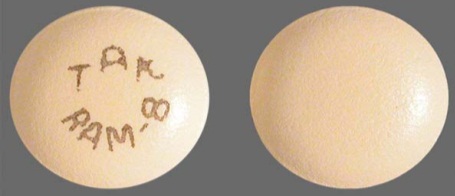
|
| Appearance | Round, pale orange-yellow, film-coated, with “TAK” and “RAM-8” printed on one side |
| Imprint code / Engraving / Debossment | “TAK” and “RAM-8” printed on one side and plain on other side |
| Score | no score |
| Color | Pale orange-yellow |
| Shape | Round |
| Dimension | 7 mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by | Takeda pharmaceuticals America, |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021782 | 1 | 6034239 | 22-Jul-19 | Y | Y | U - 674 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | Water | 900 | 10, 20, 30 and 45 | April 2, 2009 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | Discontinues | |
| UK | Discontinues | |
| US | ROZEREM | Download |
| The S-form is included. A major metabolite (M-II) is a diastereoisomer. Only the (2S,8S) is formed in humans. This metabolite has been tested separately. in monkeys. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
