| Active Ingredient | POMALIDOMIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POMALYST | (NDA # 204026) | CELGENE | CAPSULE;ORAL | 1MG, 2MG,3MG,4MG | 4MG | February 8, 2013 | February 8, 2018 | February 8, 2020 | 1 New molecular entity (NME) | S Standard review drug, O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
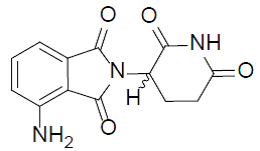 |
| Chemical Name | (RS)-4-Amino-2-(2,6-dioxo-piperidin-3-yl)-isoindoline-1,3-dione |
| CAS No | 19171-19-8 |
| Molecular Formula | C13H11N3O4 |
| Molecular Weight | 273.24 |
| Appearance | a crystalline yellow solid powder |
| Solubility | It has low solubility into organic solvents and in all pH solutions (about 0.01 mg/mL). It is slightly soluble in acetone, acetonitrile, methylene chloride, methyl ethyl ketone and tetrahydrofuran; very slightly soluble in absolute ethanol, ethyl acetate, heptane, methanol, 2-propanol and toluene; and practically insoluble in water. |
| Water Solubility | 2.57 mg/mL |
| Polymorphism | Different studies conducted have identified one single polymorphic form of pomalidomide, designated as Form A. |
| pKa (Strongest Acidic) | 11.59 (Predicted) |
| pKa (Strongest Basic) | 1.56 (Predicted) |
| Log P | 0.02 (Predicted) |
| Identification | IR |
| Degradation | - |
| Hygroscopic | non-hygroscopic |
| Photostability study | - |
| Melting Point | 315.5˚-317.5 °C |
| BCS Class | II or IV |
| Manufacture of API | The active substance is supplied by two manufacturers. Pomalidomide is synthesized in two main steps using commercially available well defined starting materials with acceptable specifications. The first step consists of a coupling reaction in which the starting materials react to form crude pomalidomide. The second step is a purification process consisting on filtration, crystallization and drying to obtain the final drug substance. As stated above, pomalidomide molecule has one stereochemical centre, which is introduced during the coupling step (step 1). The synthetic process yields a racemic mixture of pomalidomide (1:1 R:S) and a single polymorphic form, form A, is obtained. |
| Parameters | Details |
|---|---|
| Indications and Usage | POMALYST is a thalidomide analogue indicated, in combination with dexamethasone, for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy |
| Dosage and Administration |
Females of reproductive potential must have negative pregnancy testing and use contraception methods before initiating POMALYST and Use in Specific Populations. The recommended starting dose of POMALYST is 4 mg once daily orally on Days 1-21 of repeated 28-day cycles until disease progression. POMALYST should be given in combination with dexamethasone. POMALYST may be taken with water. Inform patients not to break, chew, or open the capsules. POMALYST should be taken without food (at least 2 hours before or 2 hours after a meal). |
| Mechanism of action | Pomalidomide, an analogue of thalidomide, is an immunomodulatory agent with antineoplastic activity. In in vitro cellular assays, pomalidomide inhibited proliferation and induced apoptosis of hematopoietic tumor cells. Additionally, pomalidomide inhibited the proliferation of lenalidomide-resistant multiple myeloma cell lines and synergized with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cell lines to induce tumor cell apoptosis. Pomalidomide enhanced T cell- and natural killer (NK) cell-mediated immunity and inhibited production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. Pomalidomide demonstrated anti-angiogenic activity in a mouse tumor model and in the in vitro umbilical cord model. |
| Absorption | Following administration of single oral doses of POMALYST, the maximum plasma concentration (Cmax) for pomalidomide occurs at 2 and 3 hours postdose. The systemic exposure (AUC) of pomalidomide increases in an approximately dose proportional manner. In patients with multiple myeloma who received POMALYST 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC(Τ) of 400 ng∙h/mL and Cmax of 75 ng/mL. Following multiple doses, pomalidomide has an accumulation ratio of 27% to 31%. |
| Food Effect | - |
| Distribution | Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state. Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours postdose (~Tmax) after 4 days of once-daily dosing at 2 mg. Human plasma protein binding ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-glycoprotein (P-gp). |
| Metabolism | Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. In vitro, CYP1A2 and CYP3A4 were identified as the primary enzymes involved in the CYP-mediated hydroxylation of pomalidomide, with additional minor contributions from CYP2C19 and CYP2D6. |
| Elimination |
Pomalidomide is eliminated with a median plasma half-life of approximately 9.5 hours in healthy subjects and approximately 7.5 hours in patients with multiple myeloma. Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/h. Following a single oral administration of [14C]-pomalidomide (2 mg) to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces. |
| Peak plasma time (Tmax) | 2 and 3 hours |
| Half life | 9.5 hours (median plasma half-life) |
| Bioavailability | 0.67 |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 29451 | A | II | June 6, 2015 | MYLAN LABORATORIES LTD |
| 30408 | A | II | March 30, 2016 | MSN LABORATORIES PRIVATE LTD |
| Parameters | Details | ||||
|---|---|---|---|---|---|
| Strength | 1MG | 2MG | 3MG | 4MG | |
| Excipients used | mannitol, pregelatinized starch, and sodium stearyl fumarate | ||||
| Composition of coating material | - | ||||
| Composition of caspule shell |
capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink. white ink:shellac, titanium dioxide (E171), simethicone, propylene glycol (E1520) and ammonium hydroxide (E527); black ink: shellac, iron oxide black (E172), propylene glycol (E1520) and ammonium hydroxide (E527) |
Capsule shell:gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, FD&C red 3, and white ink white ink: shellac, titanium dioxide (E171), simethicone, propylene glycol (E1520) and ammonium hydroxide (E527) |
Capsule shell:gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, and white ink white ink: shellac, titanium dioxide (E171), simethicone, propylene glycol (E1520) and ammonium hydroxide (E527) |
Capsule shell:gelatin, titanium dioxide, FD&C blue 1, FD&C blue 2, and white ink white ink:shellac, titanium dioxide (E171), simethicone, propylene glycol (E1520) and ammonium hydroxide (E527) |
|
| Pharmaceutical Development |
The aim of the pharmaceutical development was to formulate hard capsules containing 1 mg, 2 mg, 3 mg and 4mg pomalidomide per capsule, respectively. Several formulations were evaluated during development. The first formulation developed contained anhydrous lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. In an effort to improve processing, anhydrous lactose was replaced with anhydrous dibasic calcium phosphate, and other excipients were changed accordingly. The revised formulation contained pomalidomide, anhydrous dibasic calcium phosphate, pregelatinized starch, croscarmellose sodium, and sodium stearyl fumarate. Based on the development study results, the proposed formulation was selected. The proposed capsule strengths use two common blends comprised of the same excipients, varying in the proportion of drug substance and two excipients, mannitol and sodium stearyl fumarate. Pomalidomide capsules, 1 mg and 2 mg, are dose proportional and utilize a common blend. Pomalidomide capsules, 3 mg and 4 mg, are dose proportional and utilize another common blend. A simple blending and encapsulation process has been selected. The effect of the active substance particle size distribution on dissolution and content uniformity is controlled by setting appropriate specification limits. |
||||
| Manufacture of the product | The manufacturing process consists of four main steps. In brief, pomalidomide is blended with mannitol and a portion of pregelatinised starch (Blend 1), further blended with the remainder of the pregelatinised starch (Blend 2) and mixed with sodium stearyl fumarate (Blend 3) which is then filled into the hard gelatin capsule shells and packed into blisters. | ||||
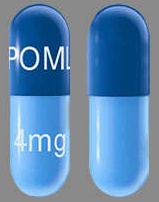
| Tablet / Capsule Image |
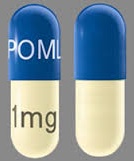
|
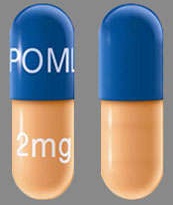
|
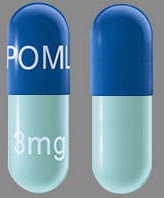
|
|
|
| Appearance | Dark blue opaque cap and yellow opaque body, imprinted “POML” on the cap in white ink and “1 mg” on the body in black ink | Dark blue opaque cap and orange opaque body, imprinted “POML” on the cap and “2 mg” on the body in white ink | Dark blue opaque cap and green opaque body, imprinted “POML” on the cap and “3 mg” on the body in white ink | Dark blue opaque cap and blue opaque body, imprinted “POML” on the cap and “4 mg” on the body in white ink | |
| Imprint code / Engraving / Debossment | imprinted “POML” on the cap in white ink and “1 mg” on the body in black ink | imprinted “POML” on the cap and “2 mg” on the body in white ink | imprinted “POML” on the cap and “3 mg” on the body in white ink | imprinted “POML” on the cap and “4 mg” on the body in white ink | |
| Score | No score | No score | No score | No score | |
| Color | YELLOW, BLUE | ORANGE, BLUE | GREEN, BLUE | BLUE, BLUE | |
| Shape | CAPSULE | CAPSULE | CAPSULE | CAPSULE | |
| Dimension | 14mm | 18mm | 18mm | 18mm | |
| Mfg by | - | ||||
| Mfg for | Celgene Corporation (US) | ||||
| Marketed by | Celgene Corporation (US, EU) | ||||
| Distributed by | - | ||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N204026 | 1 | 5635517 | July 24, 2016 | - | - | U - 1359 | - | Download |
| N204026 | 1 | 6045501 | August 28, 2018 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 6315720 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 6316471 | August 10, 2016 | - | Y | U - 1360 | - | Download |
| N204026 | 1 | 6476052 | July 24, 2016 | - | Y | U - 1360 | - | Download |
| N204026 | 1 | 6561976 | August 28, 2018 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 6561977 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 6755784 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 6908432 | August 28, 2018 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8158653 | August 10, 2016 | - | Y | - | - | Download |
| N204026 | 1 | 8198262 | October 19, 2024 | - | - | U - 1360 | - | Download |
| N204026 | 1 | 8204763 | August 28, 2018 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| N204026 | 1 | 8315886 | October 23, 2020 | - | - | U - 1361 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.1 N HCl | 900 | 10, 15, 20, 30 and 45 | May 28, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | IMNOVID | Download |
| UK | IMNOVID | Download |
| US | POMALYST | Download |
| Exclusivity Code: Exclusivity Expiration is I - 707: Apr 23, 2018. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
