| Active Ingredient | ORLISTAT |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XENICAL | 20766 | CHEPLAPHARM | CAPSULE;ORAL | 120MG | 120MG | April 23, 1999 | _ | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
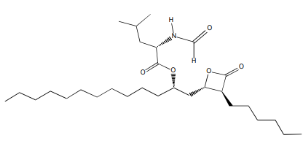 |
| Chemical Name | (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]- dodecyl ester |
| CAS No | 96829-58-2 |
| Molecular Formula | C29H53NO5 |
| Molecular Weight | 495.7 |
| Appearance | White to off-white crystalline powder |
| Solubility | Orlistat is practically insoluble in water, freely soluble in chloroform, and very soluble in methanol and ethanol. Orlistat has no pKa within the physiological pH range. |
| Water Solubility | 9.19e-05 mg/mL |
| Polymorphism | - |
| pKa (Strongest Acidic) | 12.74 |
| pKa (Strongest Basic) | -0.91 |
| Log P | 7.61, 8.11 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 40-50°C |
| BCS Class | IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | XENICAL is a reversible inhibitor of gastrointestinal lipases indicated for obesity management including weight loss and weight maintenance when used in conjunction with a reduced-calorie diet. XENICAL is also indicated to reduce the risk for weight regain after prior weight loss. |
| Dosage and Administration |
One 120-mg capsule three times a day with each main meal containing fat (during or up to 1 hour after the meal). Advise patients to take a nutritionally balanced, reduced-calorie diet that contains approximately 30% of calories from fat. Distribute the daily intake of fat, carbohydrate, and protein over three main meals. Advise patients to take a multivitamin containing fat-soluble vitamins to ensure adequate nutrition. Take the vitamin supplement at least 2 hours before or after the administration of XENICAL, such as at bedtime. For patients receiving both XENICAL and cyclosporine therapy, administer cyclosporine 3 hours after XENICAL. For patients receiving both XENICAL and levothyroxine therapy, administer levothyroxine and XENICAL at least 4 hours apart. |
| Mechanism of action | Orlistat is a reversible inhibitor of gastrointestinal lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipases. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control. |
| Absorption | Systemic exposure to orlistat is minimal. Following oral dosing with 360 mg 14C-orlistat, plasma radioactivity peaked at approximately 8 hours; plasma concentrations of intact orlistat were near the limits of detection (<5 ng/mL). In therapeutic studies involving monitoring of plasma samples, detection of intact orlistat in plasma was sporadic and concentrations were low (<10 ng/mL or 0.02 µM), without evidence of accumulation, and consistent with minimal absorption. |
| Food Effect | - |
| Distribution | In vitro orlistat was >99% bound to plasma proteins (lipoproteins and albumin were major binding proteins). Orlistat minimally partitioned into erythrocytes. |
| Metabolism | Based on an oral 14C-orlistat mass balance study in obese patients, two metabolites, M1 ((the hydrolyzed β-lactone ring product of orlistat) and M3 (sequential metabolite after M1’s cleavage of the N-formyl leucine side-chain), accounted for approximately 42% of total radioactivity in plasma. M1 and M3 have an open β-lactone ring and extremely weak lipase inhibitory activity (1000- and 2500-fold less than orlistat, respectively). In view of this low inhibitory activity and the low plasma levels at the therapeutic dose (average of 26 ng/mL and 108 ng/mL for M1 and M3, respectively, 2 to 4 hours after a dose), these metabolites are considered pharmacologically inconsequential. The primary metabolite M1 had a short half-life (approximately 3 hours) whereas the secondary metabolite M3 eliminated at a slower rate (half-life approximately 13.5 hours). |
| Elimination | Following a single oral dose of 360 mg 14C-orlistat in both normal weight and obese subjects, fecal excretion of the unabsorbed drug was found to be the major route of elimination. Orlistat and its M1 and M3 metabolites were also subject to biliary excretion. Approximately 97% of the administered radioactivity was excreted in feces; 83% of that was found to be unchanged orlistat. The cumulative renal excretion of total radioactivity was <2% of the given dose of 360 mg 14C-orlistat. The time to reach complete excretion (fecal plus urinary) was 3 to 5 days. The disposition of orlistat appeared to be similar between normal weight and obese subjects. Based on limited data, the half-life of the absorbed orlistat is in the range of 1 to 2 hours. |
| Peak plasma time (Tmax) | 8 hours |
| Half life | 1 to 2 hours. |
| Bioavailability | - |
| Age, gender | No pharmacokinetic study was conducted for specific populations such as geriatric, different races, and patients with renal and hepatic impairment. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 18315 | A | III | April 28, 2005 | NYPRO INC ORLISTAT 60 MG PORTABLE PACKAGE ® |
| 22110 | I | II | October 24, 2008 | ZHEJIANG HISUN PHARMACEUTICAL CO LTD ORLISTAT |
| 22357 | I | II | December 30, 2008 | SUN PHARMACEUTICAL INDUSTRIES LTD ORLISTAT (NON-STERILE BULK) |
| 23978 | A | II | July 19, 2010 | FORMOSA LABORATORIES INC ORLISTAT USP |
| 24885 | A | II | April 12, 2011 | CHONGQING ZEIN PHARMACEUTICAL CO LTD ORLISTAT |
| 26101 | A | II | May 18, 2012 | BIOCON LTD ORLISTAT |
| 26455 | A | II | September 21, 2012 | ARGUS PHARMACEUTICALS LTD ORLISTAT |
| 27312 | A | II | September 24, 2013 | HISUN PHARMACEUTICAL HANGZHOU CO LTD ORLISTAT USP |
| 27624 | A | II | September 23, 2013 | MURLI KRISHNA PHARMA PVT LTD ORLISTAT PELLETS 50.0% W/W |
| 29914 | A | II | October 9, 2015 | SHANDONG NEW TIME PHARMACEUTICAL CO LTD ORLISTAT |
| 30780 | A | II | August 1, 2016 | MURLI KRISHNA PHARMA PVT LTD ORLISTAT PELLETS 50.0% W/W |
| 32338 | A | II | January 31, 2018 | SUN PHARMACEUTICAL INDUSTRIES LTD ORLISTAT USP |
| Parameters | Details |
|---|---|
| Strength | 120MG |
| Excipients used | Microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, povidone,and talc |
| Composition of coating material | NA |
| Composition of caspule shell | Gelatin, titanium dioxide, and FD&C Blue No. 2 with black printing ink containing pharmaceutical grade shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonium solution, potassium hydroxide and black iron oxide. |
| Pharmaceutical Development | NA |
| Manufacture of the product | NA |
| Tablet / Capsule Image |
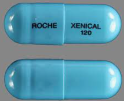
|
| Appearance | Turquoise, hard-gelatin capsule containing pellets of powder |
| Imprint code / Engraving / Debossment | Imprint “XENICAL 120” |
| Score | No score |
| Color | Turquoise |
| Shape | CAPSULE |
| Dimension | 19mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by |
Genentech USA, Inc. A Member of the roche group 1 DNA Way South San Francisco, CA 94080-4990 |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 3% SLS in 0.5% Sodium Chloride, pH 6.0 | 900 | 10, 20, 30, 45 and 60 | February 12,2004 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | XENICAL | Download |
| UK | XENICAL | Download |
| US | XENICAL | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
