| Active Ingredient | OMADACYCLINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NUZYRA | 209816 | PARATEK PHARMS INC | TABLET;ORAL | 150 MG | 150 MG | October 2, 2018 | October 2, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
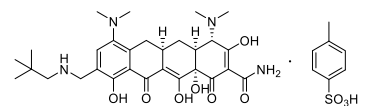 |
| Chemical Name | (4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-9-(2,2-dimethylpropylaminomethyl)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12aoctahydrotetracene-2-carboxamide,4-methylbenzenesulfonate. |
| CAS No | 1075240-43-5 |
| Molecular Formula | C36H48N4O10S (monotosylate salt) |
| Molecular Weight | 728.9 (monotosylate salt) |
| Appearance | - |
| Solubility | - |
| Water Solubility | 0.213 mg/mL |
| Polymorphism | - |
| pKa (Strongest Acidic) | 2.87 |
| pKa (Strongest Basic) | 10.54 |
| Log P | 0.94 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | NUZYRA is a tetracycline class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms : Community-acquired bacterial pneumonia (CABP) Acute bacterial skin and skin structure infections (ABSSSI) To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. |
| Dosage and Administration |
Dosage of NUZYRA in CABP and ABSSSI Adult Patients: CABP :Loading dose:Day 1: 200 mg by intravenous infusion over 60 minutes OR 100 mg by intravenous infusion over 30 minutes twice, Maintenance dose:100 mg by intravenous infusion over 30 minutes once daily OR 300 mg orally once daily ABSSSI: Loading dose: Day 1: 200 mg by intravenous infusion over 60 minutes OR 100 mg by intravenous infusion over 30 minutes twice, Maintenance dose: 100 mg by intravenous infusion over 30 minutes once daily OR 300 mg orally once daily ABSSSI (NUZYRA tablets only): Loading dose:Day 1 and Day 2: 450 mg orally once daily, Maintence dose: 300 mg orally once daily CABP and ABSSSI: Treatment duration is 7 to 14 days. Fast for at least 4 hours and then take NUZYRA tablets with water. After oral dosing, no food or drink (except water) is to be consumed for 2 hours and no dairy products, antacids, or multivitamins for 4 hours. See full prescribing information for the preparation of NUZYRA IV and other administration instructions. Tablets: 150 mg omadacycline (equivalent to 196 mg omadacycline tosylate) |
| Mechanism of action | Omadacycline is an aminomethylcycline antibacterial within the tetracycline class of antibacterial drugs. Omadacycline binds to the 30S ribosomal subunit and blocks protein synthesis.Omadacycline is active in vitro against Gram positive bacteria expressing tetracycline resistance active efflux pumps (tetK and tet L) and ribosomal protection proteins (tet M). In general, omadacycline is considered bacteriostatic; however, omadacycline has demonstrated bactericidal activity against some isolates of S. pneumoniae and H. influenzae. |
| Absorption | The exposure to omadacycline is similar between a 300-mg oral dose and a 100-mg intravenous dose of NUZYRA in healthy fasted subjects. (More details refer FDA PIL) |
| Food Effect |
Ingestion of a standard high-fat nondairy meal (855 calories; 59% calories from fat) and standard high-fat meal including dairy (985 calories; 60% calories from fat) 2-hours before administration of a single 300-mg oral dose of NUZYRA decreased the rate (Cmax) and extent of absorption (AUC) by 40% and 42%, and 59% and 63%, respectively compared to administration of NUZYRA under fasting conditions. The rate and extent of absorption of NUZYRA were not substantially decreased when a high-fat nondairy meal (800-1000 calories; 50% calories from fat) was ingested 4 hours pre-dose. Following ingestion of either a light non-fat (300-350 calories; ≤5% calories from fat), or a standard low-fat (800-1000 calories; 30% calories from fat), or a standard high fat (800-1000 calories; 50% calories from fat) meal 2 hours post-dose, the AUC and Cmax were not substantially altered, as compared to fasting conditions. |
| Distribution | Plasma protein binding of omadacycline is approximately 20% and is not concentration dependent.The mean (% CV) volume of distribution of omadacycline at steady-state following IV administration of NUZYRA in healthy subjects was 190 (27.7) L. |
| Metabolism | In vitro studies using human liver microsomes and hepatocytes demonstrated that omadacycline is not metabolized. |
| Elimination |
Renal clearance of omadacycline following IV administration of NUZYRA ranged from 2.4 to 3.3 L/h in healthy subjects. Excretion Following a 100-mg IV dose of NUZYRA, 27% of the dose was recovered as unchanged omadacycline in the urine. In healthy male volunteers receiving 300-mg oral [14C] NUZYRA, 77.5% to 84.0% of the dose was recovered in the feces, approximately 14.4 % (range 10.8% to 17.4%) in the urine, with 95.5% of the administered radioactive dose recovered after 7 days. |
| Peak plasma time (Tmax) | 2.50 Hours |
| Half life | 300 mg Oral Single dose: 14.96 (16.5) hours Steady state:15.5 (10.7) hours 450 mg Oral Single dose: 13.45 (12.9) hours Steady state:16.83 (8.1) hours |
| Bioavailability | 34.5% following single 300 mg dose of NUZYRA |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 150 MG |
| Excipients used |
Colloidal silicon dioxide, crospovidone, lactose monohydrate, microcrystalline cellulose, sodium bisulfite, sodium stearyl fumarate |
| Composition of coating material | Glycerol monocaprylocaprate, iron oxide yellow,polyvinyl alcohol,sodium lauryl sulfate, talc, and titanium dioxide. |
| Composition of caspule shell | NA |
| Pharmaceutical Development |
NUZYRA (omadacycline) tablets for oral administration are yellow film coated tablets containing 150 mg of omadacycline (equivalent to 196 mg omadacycline tosylate) |
| Manufacture of the product | Updated soon |
| Tablet / Capsule Image | |
| Appearance | Yellow, diamond-shaped, film-coated tablets debossed with OMC on one side and 150 on the other side. |
| Imprint code / Engraving / Debossment | Debossed with OMC on one side and 150 on the other side |
| Score | No score |
| Color | Yellow |
| Shape | Diamond |
| Dimension | 19 mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by |
Paratek Pharmaceuticals, Inc. Boston, MA, USA |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N209816 | 1 | 10111890 | August 3, 2037 | - | - | U-2444 | - | |
| N209816 | 1 | 10124014 | March 5, 2029 | - | - | U-2449 | - |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II | 60 | 0.1 N HCL | 900 mL | 85% in 15 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
