| Active Ingredient | NITAZOXANIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALINIA | (NDA): 021497 | ROMARK | TABLET;ORAL | 500 MG | 500 MG | July 21, 2004 | _ | _ | Type 3 - New Dosage Form | PRIORITY ; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
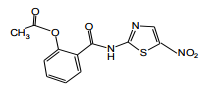 |
| Chemical Name | 2-acetyloxy-N-(5-nitro-2-thiazolyl)benzamide |
| CAS No | 55981-09-4 |
| Molecular Formula | C12H9N3O5S |
| Molecular Weight | 307.3. |
| Appearance | Light yellow crystalline powder |
| Solubility | Poorly soluble in ethanol |
| Water Solubility | Practically insoluble in water |
| Polymorphism | - |
| pKa (Strongest Acidic) | 8.3 |
| pKa (Strongest Basic) | -4.2 |
| Log P | 1.2 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 202 °C |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | ALINIA is an antiprotozoal indicated for the treatment of diarrhea caused by Giardia lamblia or Cryptosporidium parvum. Limitations of Use: ALINIA has not been shown to be effective for the treatment of diarrhea caused by C. parvum in HIV-infected or immunodeficient patients |
| Dosage and Administration |
ALINIA Tablets should not be administered to pediatric patient 11 years of age or younger. Dosage for treatment of diarrhea caused by G. lamblia or C. parvum. Age Dosage Duration 1-3 years 5 mL of ALINIA for Oral Suspension (100 mg nitazoxanide) every 12 hours with food 4-11 years 10 mL of ALINIA for Oral Suspension (200 mg nitazoxanide) every 12 hours with food 3 days 12 years and older One ALINIA Tablet (500 mg nitazoxanide) every 12 hours with food or 25 mL of ALINIA for Oral Suspension (500 mg nitazoxanide) every 12 hours with food |
| Mechanism of action |
The antiprotozoal activity of nitazoxanide is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. Studies have shown that the PFOR enzyme from G. lamblia directly reduces nitazoxanide by transfer of electrons in the absence of ferredoxin. The DNA-derived PFOR protein sequence of C. parvum appears to be similar to that of G. lamblia. Interference with the PFOR enzyme-dependent electron transfer reaction may not be the only pathway by which nitazoxanide exhibits antiprotozoal activity. |
| Absorption |
Single Dosing: Following oral administration of ALINIA Tablets or Oral Suspension, the parent drug, nitazoxanide, is not detected in plasma. Multiple dosing: Following oral administration of a single ALINIA Tablet every 12 hours for 7 consecutive days,there was no significant accumulation of nitazoxanide metabolites tizoxanide or tizoxanide glucuronide detected in plasma. Refer FDA PIL for more details |
| Food Effect |
When ALINIA Tablets are administered with food, the AUC of tizoxanide and tizoxanide glucuronide in plasma is increased almost two-fold and the Cmax is increased by almost 50%. When ALINIA for Oral Suspension was administered with food, the AUC of tizoxanide and tizoxanide glucuronide increased by about 45-50% and the Cmax increased by 10%. ALINIA Tablets and ALINIA for Oral Suspension were administered with food in clinical trials and hence they are recommended to be administered with food |
| Distribution | In plasma, more than 99% of tizoxanide is bound to proteins. |
| Metabolism | Following oral administration in humans, nitazoxanide is rapidly hydrolyzed to an active metabolite, tizoxanide (desacetyl-nitazoxanide). Tizoxanide then undergoes conjugation, primarily by glucuronidation |
| Elimination | Tizoxanide is excreted in the urine, bile and feces, and tizoxanide glucuronide is excreted in urine and bile. Approximately two-thirds of the oral dose of nitazoxanide is excreted in the feces and onethird in the urine. |
| Peak plasma time (Tmax) | Tizoxanide : 3 to 6 hrs (based on the age) Tizoxanide Glucuronide : 4 hrsx |
| Half life | - |
| Bioavailability | ALINIA for Oral Suspension is not bioequivalent to ALINIA Tablets. The relative bioavailability of the suspension compared to the tablet was 70%. |
| Age, gender | The pharmacokinetics of tizoxanide and tizoxanide glucuronide following administration of ALINIA Tablets in pediatric patients 12-17 years of age are provided in Table 2 (FDA PIL). The pharmacokinetics of tizoxanide and tizoxanide glucuronide following administration of ALINIA for Oral Suspension in pediatric patients 1-11 years of age are provided above in Table 3 (FDA PIL) |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 12124 | A | II | September 4, 1996 | LAPICOR BVBA |
| 19752 | A | II | September 8, 2006 | SUVEN LIFE SCIENCES LTD |
| 23792 | A | II | May 6, 2010 | INDUSTRIALE CHIMICA SRL |
| 31541 | A | II | December 6, 2016 | CAMBREX PROFARMACO MILANO SRL |
| Parameters | Details |
|---|---|
| Strength | 500 MG |
| Excipients used | Maize starch (190.20 mg) pregelatinized corn starch (63.4 mg), hydroxypropyl methylcellulose (5 mg),sodium starch glycollate (40 mg), talc, magnesium stearate (6.4 mg) |
| Composition of coating material | Sucrose, talc soy lecithin, polyvinyl alcohol, anthan gum, itanium dioxide, FD&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, and FD&C Blue No. 2 Aluminum Lake. |
| Composition of caspule shell | - |
| Pharmaceutical Development | - |
| Manufacture of the product | - |
| Tablet / Capsule Image |
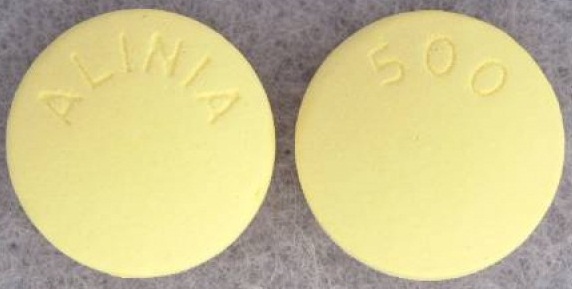
|
| Appearance | Round, yellow, film-coated tablets debossed with ALINIA on one side and 500 on the other side |
| Imprint code / Engraving / Debossment | Debossed with ALINIA on one side and 500 on the other side |
| Score | No score |
| Color | Yellow |
| Shape | Round |
| Dimension | 13 mm |
| Mfg by | Romark, L.C. |
| Mfg for | - |
| Marketed by | - |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021497 | 1 | 5968961 | May 7, 2017 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | Phosphate buffer at pH 7.5 with 6% hexadecyltrimethyl ammonium bromide, bath temperature at 25ºC | 900 | 10, 20, 30, 45, 60 | 01/03/2007 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
