| Active Ingredient | NILOTINIB HYDROCHLORIDE MONOHYDRATE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TASIGNA | (NDA) 022068 | NOVARTIS | CAPSULE;ORAL | EQ 200MG BASE , EQ 150MG BASE | EQ 200MG BASE | October 29, 2007 | _ | _ | 1 New molecular entity (NME) | S Standard review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
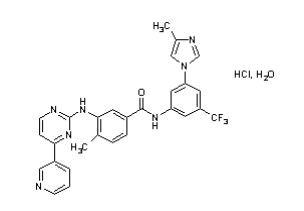 |
| Chemical Name | 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-3[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-benzamide, monohydrochloride, monohydrate |
| CAS No | 641571-10-0 |
| Molecular Formula | C28H22F3N7O•HCl • H2O |
| Molecular Weight | 583.99 (as monohydrate) and 565.98 (as anhydrate) |
| Appearance | White to slightly yellowish to slightly greenish yellow powderx |
| Solubility | The solubility of nilotinib hydrochloride monohydrate in aqueous solutions at 25°C strongly decreases with increasing pH, and it is practically insoluble inbuffer solutions of pH 4.5 and higher pH values. Nilotinib is sparingly soluble in ethanol and methanol.Active substance is slightly soluble at pH 1 but practically insoluble at pH 6.8 |
| Water Solubility | 0.00201 mg/mL |
| Polymorphism | The different crystalline Forms A, B, C and amorphous have been characterized. Form A corresponds to a dihydrate form. Form B and Form C are monohydrate forms obtained after desolvation of different solvates. Form B, isolated from the synthetic process and used in the medicinal product, is the most stable form. It shows the least hygroscopic behaviour of all the three crystalline forms A, B, and C. No transformation was observed after storing these three forms at room temperature even for several months |
| pKa (Strongest Acidic) | The pKa1 was determined to be 2.1; pKa2 was estimated to be 5.4. |
| pKa (Strongest Basic) | 6.3 |
| Log P | 4.51 (The distribution coefficient (D) for nilotinib hydrochloride monohydrate in n-octanol / 0.1 N HCl buffer at 37.0 ± 0.5 °C was determined to be 0.08, and the corresponding Log D -1.1) |
| Identification | IR, X-ray diffraction |
| Degradation | Slight hydrolysisof the active substance observed |
| Hygroscopic | Slight hygroscopicity |
| Photostability study | Slightly light sensitive |
| Melting Point | - |
| BCS Class | IV |
| Manufacture of API | The procedure for the manufacture of nilotinib hydrochloride monohydrate involves four synthetic transformations and one sieving step. Reprocessing may take place according to the above procedure, starting at an appropriate stage. |
| Parameters | Details |
|---|---|
| Indications and Usage | Tasigna is a kinase inhibitor indicated for the treatment of: Adult and pediatric patients greater than or equal to 1 year of age with newly diagnosed Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in chronic phase. Adult patients with chronic phase (CP) and accelerated phase (AP) Ph+CML resistant to or intolerant to prior therapy that included imatinib. Pediatric patients greater than or equal to 1 year of age with Ph+ CML-CP resistant or intolerant to prior tyrosine-kinase inhibitor (TKI) therapy. |
| Dosage and Administration |
Recommended Adult Dose: Newly diagnosed Ph+ CML-CP: 300 mg orally twice daily. Resistant or intolerant Ph+ CML-CP and CML-AP: 400 mg orally twice daily. Recommended Pediatric Dose: Newly Diagnosed Ph+ CML-CP or Ph+ CML-CP resistant or intolerant to prior TKI therapy: 230 mg/m2 orally twice daily, rounded to the nearest 50 mg dose (to a maximum single dose of 400 mg). See Dosage and Administration (2.1) for full dosing instructions and dosereduction instructions for toxicity. Reduce starting dose in patients with baseline hepatic impairment. Eligible newly diagnosed adult patients with Ph+ CML-CP who have received Tasigna for a minimum of 3 years and have achieved a sustained molecular response (MR4.5) and patients with Ph+ CML-CP resistant or intolerant to imatinib who have received Tasigna for at least 3 years and have achieved a sustained molecular response (MR4.5) may be considered for treatment discontinuation |
| Mechanism of action | Nilotinib is an inhibitor of the BCR-ABL kinase. Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. In vitro, nilotinib inhibited BCR-ABL mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ CML. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from BCR-ABL kinase mutations, in 32 out of 33 mutations tested. In vivo, nilotinib reduced the tumor size in a murine BCR-ABL xenograft model. Nilotinib inhibited the autophosphorylation of the following kinases at IC50 values as indicated: BCR-ABL (20 to 60 nM), PDGFR (69 nM), c-KIT (210 nM), CSF-1R (125 to 250 nM), and DDR1 (3.7 nM). |
| Absorption |
Steady-state nilotinib exposure was dose-dependent with less than dose-proportional increases in systemic exposure at dose levels higher than 400 mg given as once-daily dosing. Daily serum exposure to nilotinib following 400 mg twice-daily dosing at steady state was 35% higher than with 800 mg once-daily dosing. Steady state exposure (AUC) of nilotinib with 400 mg twice-daily dosing was 13% higher than with 300 mg twice-daily dosing. The average steady state nilotinib trough and peak concentrations did not change over 12 months. There was no relevant increase in exposure to nilotinib when the dose was increased from 400 mg twice-daily to 600 mg twice-daily Single dose administration of two 200 mg nilotinib capsules each dispersed in 1 teaspoon of applesauce and administered within 15 minutes was shown to be bioequivalent to a single dose administration of two 200 mg intact capsules. The blood-to-serum ratio of nilotinib is 0.68. Serum protein binding is approximately 98% on the basis of in vitro experiments. |
| Food Effect |
The bioavailability of nilotinib is increased with food, thus Tasigna must not be taken with food. No food should be consumed for at least 2 hours before and for at least 1 hour after the dose is taken. Also avoid grapefruit products and other foods that are known to inhibit CYP3A4. The bioavailability of nilotinib was increased when given with a meal. Compared to the fasted state, the systemic exposure (AUC) increased by 82% when the dose was given 30 minutes after a high fat meal. Median steady-state trough concentration of nilotinib was decreased by 53% in patients with total gastrectomy compared to patients who had not undergone surgeries |
| Distribution | - |
| Metabolism | The apparent elimination half-life estimated from the multiple dose pharmacokinetic studies with daily dosing was approximately 17 hours. Inter-patient variability in nilotinib AUC was 32% to 64%. Steady state conditions were achieved by Day 8. An increase in serum exposure to nilotinib between the first dose and steady state was approximately 2-fold for daily dosing and 3.8-fold for twice-daily dosing. Main metabolic pathways identified in healthy subjects are oxidation and hydroxylation. Nilotinib is the main circulating component in the serum. None of the metabolites contribute significantly to the pharmacological activity of nilotinib. After a single dose of radiolabeled nilotinib in healthy subjects, more than 90% of the administered dose was eliminated within 7 days: mainly in feces (93% of the dose). Parent drug accounted for 69% of the dose. |
| Elimination | - |
| Peak plasma time (Tmax) | 3 hours after oral administration |
| Half life | 17 hours |
| Bioavailability | Relative bioavailability of nilotinib capsule is approximately 50% |
| Age, gender | Age, body weight, gender, or ethnic origin did not significantly affect the pharmacokinetics of nilotinib |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 25338 | A | II | September 28, 2011 | ZHEJIANG JIUZHOU PHARMACEUTICAL CO LTD NILOTINIB HYDROCHLORIDE MONOHYDRATE (FORM B) (VERSION NO.: 1) |
| 25412 | A | II | October 19, 2011 | ZHEJIANG JIUZHOU PHARMACEUTICAL CO LTD NILOTINIB HYDROCHLORIDE MONOHYDRATE (VERSION NO.: 1) |
| 26497 | I | II | September 28, 2012 | SICHUAN XIELI PHARMACEUTICAL CO LTD NILOTINIB |
| 28102 | A | II | July 15, 2014 | TEVA PHARMACEUTICAL INDUSTRIES LTD NILOTINIB HYDROCHLORIDE |
| 30088 | A | II | December 31, 2015 | DR REDDYS LABORATORIES LTD NILOTINIB HYDROCHLORIDE |
| 30098 | A | II | December 9, 2015 | HETERO LABS LTD NILOTINIB HYDROCHLORIDE DIHYDRATE |
| 30164 | A | II | December 29, 2015 | HIKMA PHARMACEUTICALS PLC NILOTINIB MONOHYDROCHLORIDE ANHYDROUS |
| 31693 | A | II | April 14, 2017 | SUZHOU LIXIN PHARMACEUTICAL CO LTD NILOTINIB HYDROCHLORIDE MONOHYDRATE |
| 31740 | A | II | June 30, 2017 | FIS FABBRICA ITALIANA SINTETICI SPA NILOTINIB DIHYDROCHLORIDE DIHYDRATE |
| Parameters | Details | ||
|---|---|---|---|
| Strength | EQ 200MG BASE | EQ 150MG BASE | |
| Excipients used | Colloidal silicon dioxide (2.10 mg), crospovidone (15.91)mg, lactose monohydrate( 156.11 mg), magnesium stearate (2.10 mg) poloxamer 188 (3.18 mg) |
Colloidal silicon dioxide (1.58 mg), crospovidone (11.93)mg, lactose monohydrate(117.08 mg), magnesium stearate (1.58 mg) poloxamer 188 (2.39 mg) |
|
| Composition of coating material | NA | ||
| Composition of caspule shell | Gelatin (94.87 mg), iron oxide (yellow) (0.34 mg), iron oxide (black) (0.17) mg, and titanium dioxide (0.96 mg). | Gelatin (74.54 mg), iron oxide (red) (0.34 mg), iron oxide (black) (0.36 mg), and titanium dioxide (0.76 mg). | |
| Pharmaceutical Development |
The objective of the development was to develop animmediate release solid dosage form for oral administration by taking the following prerequisites into account: • High dose to be administered (maximum daily dose = 1200 mg of active substance) • Active substance is slightly soluble at pH 1 but practically insoluble at pH 6.8 • Tendency of active substance to agglomeration and electrostatic ehaviour, low bulk density of active substance From the existing polymorphic forms, form B, a monohydrate form of nilotinib hydrochloride, has been selected for development and has been used over the toxicity and clinical program. It shows least hygroscopic behaviour among the existing crystalline forms. In development of the active substance no transformation of the polymorphic form has been observed The impact of the particle size on the drug release kinetics has been assessed bytesting the dissolution rate of product manufactured with active substance of different particle sizes. It could be observed that the particle size has no impact on the dissolution characteristics of the medicinal product. Based on these results the limits for particle size were set on technological considerations only, i.e. to limit the amount of coarse particles of the active substance and thus to ensure an appropriate content uniformity of the medicinal product and to control the electrostatic behaviour of the active substance resulting in acceptable flow properties of the final blend. The particle size of the sieved active substance is routinely determined by laser light diffraction |
||
| Manufacture of the product | Aqueous wet granulation, drying, screening, mixing and encapsulation. Therefore manufacturing process development was focused on the evaluation of critical process parameters. | ||
| Tablet / Capsule Image |
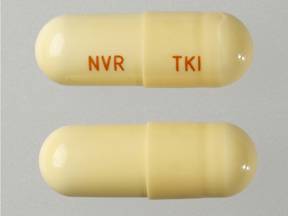
|
||
| Appearance | Light yellow opaque hard gelatin capsules, size 0 with the red axial imprint “NVR/TKI.” | Red opaque hard gelatin capsules, size 1 with black axial imprint “NVR/BCR.” | |
| Imprint code / Engraving / Debossment | “NVR/TKI.” | “NVR/BCR.” | |
| Score | No score | No score | |
| Color | Light yellow opaque | Red opaque | |
| Shape | Capsule-shape | Capsule-shape | |
| Dimension | 22 mm | 22 mm | |
| Mfg by | Novartis Pharmaceuticals | ||
| Mfg for | - | ||
| Marketed by |
Novartis Europharm Limited Frimley Business Park Camberley GU16 7SR United Kingdom |
||
| Distributed by |
Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936 |
||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N022068 | 2 | 7169791 | July 4, 2023 | Y | Y | U - 836 | - | Download |
| N022068 | 2 | 8163904 | August 23, 2028 | Y | Y | - | - | Download |
| N022068 | 2 | 8293756 | September 25, 2027 | - | Y | - | - | Download |
| N022068 | 2 | 8389537 | July 18, 2026 | Y | Y | U - 1374 | - | Download |
| N022068 | 2 | 8415363 | July 18, 2026 | Y | Y | U - 1407 | - | Download |
| N022068 | 2 | 8501760 | July 18, 2026 | Y | Y | - | - | Download |
| N022068 | 2 | 9061029 | 48311 | - | Y | U-1374 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| I (Basket) | 100 | 0.1 N HCl | 1000 | 10, 15, 30 and 45 | October 30, 2009 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| Canada | TASIGNA (NOVARTIS PHARMACEUTICALS CANADA INC) | |
| EU | TASIGNA | Download |
| South Africa | TASIGNA (Novartis South Africa (Pty) Ltd)-200 mg only | |
| UK | TASIGNA | Download |
| US | TASIGNA | Download |
| 150 mg capsule- xOne hard capsule contains 117.08 mg lactose (as monohydrate). One hard capsule contains 156.11 mg lactose (as monohydrate). Single-dose administration of 400 mg nilotinib, using 2 hard capsules of 200 mg whereby the content of each hard capsule was dispersed in one teaspoon of apple sauce, was shown to be bioequivalent with a single-dose administration of 2 intact hard capsules of 200 mg. Stability: At accelerated storage conditions gelatin shell cross linking was also observed. For some samples cross linking could not be reversed by addition of pepsin and thus did not comply with the specifications. The cross linking of the gelatin shell (i.e. formation of water insoluble membrane during dissolution acting as a barrier restricting drug release) is a well known phenomenon observed when hard gelatin capsules are stressed by high temperature and humidity. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
