| Active Ingredient | METHYLNALTREXONE BROMIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RELISTOR | (NDA) 208271 | SALIX PHARMS INC | TABLET;ORAL | 150MG | 150MG | July 19, 2016 | _ | _ | 3 New dosage form | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
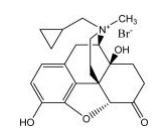 |
| Chemical Name | (R)-N-(cyclopropylmethyl) noroxymorphone methobromide |
| CAS No | 916055-93-1 |
| Molecular Formula | C21H26NO4Br |
| Molecular Weight | 436.36 |
| Appearance | White to off-white crystalline powder |
| Solubility | Very slightky soluble in alcohols |
| Water Solubility | Soluble in water and aqueous buffer (pH 1 to 12) |
| Polymorphism | - |
| pKa (Strongest Acidic) | 8.4 |
| pKa (Strongest Basic) | -3.9 (Predicted) |
| Log P | 1.12 |
| Identification | IR and HPLC |
| Degradation | - |
| Hygroscopic | Non-hygroscopic |
| Photostability study | - |
| Melting Point | 251° C |
| BCS Class | - |
| Manufacture of API | MTNX is manufactured by a synthetic process. Details on the manufacturing process, control of materials, critical steps, process controls, and process validation were provided in the restricted part of the Active Substance Master File. |
| Parameters | Details |
|---|---|
| Indications and Usage | • RELISTOR is an opioid antagonist. RELISTOR tablets and RELISTOR injection are indicated for the treatment of opioid-induced constipation (OIC) in adults with chronic non-cancer pain. • RELISTOR injection is indicated for the treatment of OIC in adults with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient. o Limitations of Use: Use beyond four months has not been studied in the advanced illness population. |
| Dosage and Administration |
Administration Instruction • Be within close proximity to toilet facilities once administered. • Discontinue if treatment with opioid pain medication is also discontinued. • In adult patients with chronic non-cancer pain and OIC: o RELISTOR has been shown to be efficacious in patients who have taken opioids for at least 4 weeks. o Discontinue all maintenance laxative therapy before starting RELISTOR; may resume laxatives if there is a suboptimal response to RELISTOR after 3 days. o Re-evaluate the continued need for RELISTOR when opioid regimen is changed to avoid adverse reactions. • In patients with chronic non-cancer pain and OIC, take RELISTOR tablets with water on an empty stomach at least 30 minutes before the first meal of the day. Dosing • For OIC in adult patients with chronic non-cancer pain: o RELISTOR tablets: The recommended dosage is 450 mg once daily in the morning. o RELISTOR injection: The recommended dosage is 12 mg subcutaneously once daily. • For OIC in adult patients with advanced illness : o The pre-filled syringe is only for patients who require a RELISTOR injection dose of 8 mg or 12 mg. Use the vial for patients who require other doses of RELISTOR injection o RELISTOR injection: See Table 1 in the full prescribing information for the recommended dosage; administer one dose every other day, as needed, but no more frequently than one dose in a 24-hour period. |
| Mechanism of action | Methylnaltrexone is a selective antagonist of opioid binding at the mu-opioid receptor. As a quaternary amine, the ability of methylnaltrexone to cross the blood-brain barrier is restricted. This allows methylnaltrexone to function as a peripherally-acting mu-opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids without impacting opioid-mediated analgesic effects on the central nervous system. |
| Absorption |
Following administration of a single 450-mg dose of RELISTOR tablets in OIC patients or healthy subjects, peak concentrations (Cmax) of methylnaltrexone were observed at approximately 1.5 hours. The absolute bioavailability of oral methylnaltrexone bromide has not been determined. The Cmax and AUC in healthy subjects were 48.1 ng/mL and 382 ng·hr/mL, respectively, following a single 450-mg dose of RELISTOR tablets. Exposure in the OIC patient population was approximately 27% lower than in healthy subjects. |
| Food Effect | Administration of a single 450-mg dose of RELISTOR tablets to healthy subjects with a high fat breakfast (containing approximately 800 to 1000 total calories, with 60%, 25% and 15% of calories derived from fat, carbohydrate and protein, respectively) resulted in a decrease in the Cmax of methylnaltrexone by 60%, the AUC by 43% and delayed the Tmax by 2 hours |
| Distribution | The steady-state volume of distribution (Vss) of methylnaltrexone is approximately 1.1 L/kg. The fraction of methylnaltrexone bound to human plasma proteins is 11% to 15%, as determined by equilibrium dialysis. |
| Metabolism |
In an intravenous mass balance study, approximately 44% of the administered radioactivity was recovered in the urine over 24 hours with 5 distinct metabolites. None of the detected metabolites was in amounts over 6% of administered radioactivity. Conversion to methyl-6-naltrexol isomers (5% of total) and methylnaltrexone sulfate (1% of total) appear to be the primary pathways of metabolism. N-demethylation of methylnaltrexone to produce naltrexone is not significant. Systemic exposure of methylnaltrexone metabolites after oral administration of a single 450-mg dose of RELISTOR tablets are greater than the systemic exposure of methylnaltrexone metabolites after subcutaneous administration of a single 12 mg dose of RELISTOR injection. Subcutaneous administration is not subject to first-pass hepatic metabolism prior to appearance in the systemic circulation. After 12 mg subcutaneous once daily dosing the mean AUC0-24 ratio of metabolites to methylnaltrexone at steady-state was 30%, 19%, and 9% for methylnaltrexone sulfate, methyl-6α-naltrexol, and methyl-6β-naltrexol, respectively. After 450 mg oral once daily dosing, the ratio of the mean AUC0-24 of metabolites to methylnaltrexone at steady-state was 79%, 38%, and 21% for methylnaltrexone sulfate, methyl-6α-naltrexol, and methyl-6β-naltrexol, respectively. Methylnaltrexone sulfate is a weak mu-opioid receptor antagonist; methyl-6α-naltrexol, and methyl-6β-naltrexol are active mu-opioid receptor antagonists. Methylnaltrexone is conjugated by sulfotransferase SULT1E1 and SULT2A1 isoforms to methylnaltrexone sulfate. Conversion to methyl-6-naltrexol isomers is mediated by aldo keto reductase 1C enzymes. |
| Elimination |
Following oral administration of a single 450-mg dose of RELISTOR tablets, concentrations of methylnaltrexone declined in multiphasic manner with a terminal half-life (t1/2) of approximately 15 hours. In an intravenous mass balance study, approximately half of the dose was excreted in the urine (54%) and 17% of administered dose was excreted in the feces up to 168 hours postdose; however, radiolabeled recovery in this study was only 71% after 7 days. Methylnaltrexone is excreted primarily as the unchanged drug in the urine and feces. Active renal secretion of methylnaltrexone is suggested by renal clearance of methylnaltrexone that is approximately 4 to 5 fold higher than creatinine clearance. No mass balance clinical studies were conducted with oral administration of methylnaltrexone bromide. However, following once daily dosing of 450 mg RELISTOR tablets for one week, the percentage of dose recovered in the urine as the parent methylnaltrexone was low (approximately 1% on both Day 1 and Day 7). |
| Peak plasma time (Tmax) | Approximately 1.5 hours |
| Half life | 15 hours |
| Bioavailability | - |
| Age, gender | A study was conducted to characterize the pharmacokinetics of methylnaltrexone after a single dose of 24 mg methylnaltrexone bromide via intravenous infusion over 20 min in healthy adults between 18 and 45 years of age and in healthy adults aged 65 years and older. In elderly subjects (mean age 72 years old), mean clearance was about 20% lower (56 L/h versus 70 L/h) and AUC∞ was 26% higher than in subjects between 18 and 45 years of age (mean age 30 years old) |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 22553 | A | II | February 16, 2009 | MALLINCKRODT INC |
| 26528 | A | II | September 14, 2012 | NORAMCO GMBH |
| Parameters | Details |
|---|---|
| Strength | 150 MG |
| Excipients used | Silicified microcrystalline cellulose, microcrystalline cellulose, sodium lauryl sulfate, croscarmellose sodium, crospovidone, poloxamer 407, stearic acid (vegetable source), colloidal silicon dioxide, edetate calcium disodium |
| Composition of coating material | Polyvinyl alcohol, titanium dioxide, polyethylene glycol and talc. |
| Composition of caspule shell | - |
| Pharmaceutical Development | 150 mg of methylnaltrexone bromide (equivalent to 122.5 mg methylnaltrexone) |
| Manufacture of the product | - |
| Tablet / Capsule Image | |
| Appearance | White, round, biconvex, and debossed with “REL” on one side and plain on the other side |
| Imprint code / Engraving / Debossment | Debossed with “REL” on one side and plain on the other side |
| Score | 2 pieces |
| Color | White |
| Shape | Round, biconvex |
| Dimension | 6 mm |
| Mfg by | - |
| Mfg for |
Salix Pharmaceuticals Lic. From: Progenics Pharmaceuticals |
| Marketed by | - |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| (NDA) 208271 | 1 | 6559158 | November 3, 2017 | - | - | U-1185 | - | Download |
| (NDA) 208271 | 1 | 8420663 | September 30, 2029 | - | - | U-1185 | - | Download |
| (NDA) 208271 | 1 | 8524276 | March 10, 2031 | - | DP | - | - | Download |
| (NDA) 208271 | 1 | 8956651 | March 10, 2031 | - | DP | - | - | Download |
| (NDA) 208271 | 1 | 9180125 | September 30, 2029 | - | DP | U-1185 | - | Download |
| (NDA) 208271 | 1 | 9314461 | March 10, 2031 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.1 N HCl | 1000 | 5, 10, 15, 20 and 30 | 10/20/2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| NP Exclusivity Expiration on Jul 19, 2019. RELISTOR subcutaneous injection available in EU, US and canada. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
