| Active Ingredient | MESALAMINE TABLET, DELAYED RELEASE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIALDA | NDA#022000 | SHIRE | TABLET, DELAYED RELEASE;ORAL | 1.2GM | 1.2GM (RS) | January 16, 2007 | - | - | Type 3 - New Dosage Form | STANDARD ; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
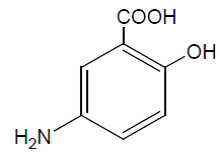 |
| Chemical Name | 5-aminosalicylic acid (5-ASA; mesalamine) |
| CAS No | 89-57-6 |
| Molecular Formula | C7H7NO3 |
| Molecular Weight | 153.14 |
| Appearance | white to light pink/grey/brown powder or crystals |
| Solubility | It is very slightly soluble in water, practically insoluble in acetone, alcohol, and ether. The drug substance dissolves in dilute solutions of alkali hydroxides and in dilute hydrochloric acid. |
| Water Solubility | 0.84 g/L at 20°C |
| Polymorphism | - |
| pKa (Strongest Acidic) | 2.02 |
| pKa (Strongest Basic) | 5.87 |
| Log P | 1.2 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 283 °C |
| BCS Class | II/III |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | LIALDA is indicated for the induction of remission in patients with active, mild to moderate ulcerative colitis and for the maintenance of remission of ulcerative colitis. |
| Dosage and Administration | The recommended dosage for the induction of remission in adult patients with active, mild to moderate ulcerative colitis is two to four 1.2 g tablets taken once daily with a meal for a total daily dose of 2.4 g or 4.8 g. The recommended dosage for the maintenance of remission is two 1.2 g tablets taken once daily with a meal for a total daily dose of 2.4 g. |
| Mechanism of action |
The mechanism of action of mesalamine is not fully understood, but appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon. Mesalamine has the potential to inhibit the activation of nuclear factor kappa B (NFкB) and consequently the production of key pro-inflammatory cytokines. It has been proposed that reduced expression of PPARγ nuclear receptors (γ-form of peroxisome proliferator-activated receptors) may be implicated in ulcerative colitis. There is evidence that mesalamine produces pharmacodynamic effects through direct activation of PPARγ receptors in the colonic/rectal epithelium. |
| Absorption |
The total absorption of mesalamine from LIALDA 2.4 g or 4.8 g given once daily for 14 days to healthy volunteers was found to be approximately 21-22% of the administered dose. Gamma-scintigraphy studies have shown that a single dose of LIALDA 1.2 g (one tablet) passed intact through the upper gastrointestinal tract of fasted healthy volunteers. Scintigraphic images showed a trail of radio-labeled tracer in the colon, suggesting that mesalamine had distributed through this region of the gastrointestinal tract. In a single dose study, LIALDA 1.2 g, 2.4 g and 4.8 g were administered in the fasted state to healthy subjects. Plasma concentrations of mesalamine were detectable after 2 hours and reached a maximum by 9-12 hours on average for the doses studied. The pharmacokinetic parameters are highly variable among subjects (Table 3). Mesalamine systemic exposure in terms of area under the plasma concentration-time curve (AUC) was slightly more than dose proportional between 1.2 g and 4.8 g LIALDA. Maximum plasma concentrations (Cmax) of mesalamine increased approximately dose proportionately between 1.2 g and 2.4 g and sub-proportionately between 2.4 g and 4.8 g LIALDA, with the dose normalized value at 4.8 g representing, on average, 74% of that at 2.4 g based on geometric means. |
| Food Effect | Administration of a single dose of LIALDA 4.8 g with a high fat meal resulted in further delay in absorption, and plasma concentrations of mesalamine were detectable 4 hours following dosing. However, a high fat meal increased systemic exposure of mesalamine (mean Cmax: Increased 91%; mean AUC: Increased 16%) compared to results in the fasted state. LIALDA was administered with food in the controlled clinical trials that supported its approval. |
| Distribution | Mesalamine is approximately 43% bound to plasma proteins at the concentration of 2.5 μg/mL. |
| Metabolism | The only major metabolite of mesalamine (5-aminosalicylic acid) is N-acetyl-5-aminosalicylic acid. Its formation is brought about by N-acetyltransferase (NAT) activity in the liver and intestinal mucosa cells, principally by NAT-1. |
| Elimination | Elimination of mesalamine is mainly via the renal route following metabolism to N-acetyl-5aminosalicylic acid (acetylation). However, there is also limited excretion of the parent drug in urine. Of the approximately 21-22% of the dose absorbed, less than 8% of the dose was excreted unchanged in the urine after 24 hours, compared with greater than 13% for N-acetyl-5-aminosalicylic acid. The apparent terminal half-lives for mesalamine and its major metabolite after administration of LIALDA 2.4 g and 4.8 g were, on average, 7-9 hours and 8-12 hours, respectively. |
| Peak plasma time (Tmax) | 9.0 hr |
| Half life | 7-9 hours (mesalamine) and 8-12 hours (its major metabolite) |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 11807 | A | II | January 15, 1996 | CORDEN PHARMA BERGAMO SPA |
| 11951 | A | II | April 29, 1996 | CHEMI SPA |
| 13883 | A | II | December 3, 1998 | ERREGIERRE SP |
| 16431 | I | II | February 17, 2003 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 18054 | I | II | February 2, 2005 | SIMS SRL |
| 18387 | I | II | May 27, 2005 | DR REDDYS LABORATORIES LTD |
| 18742 | A | II | September 9, 2005 | INFAR SA |
| 19261 | A | II | April 18, 2006 | IPCA LABORATORIES LTD |
| 19618 | A | II | July 21, 2006 | SYNTESE AS |
| 19993 | A | II | November 20, 2006 | CORDEN PHARMA BERGAMO SPA |
| 21489 | A | II | March 28, 2008 | LUPIN LTD |
| 22617 | A | II | April 3, 2010 | IPCA LABORATORIES LTD |
| 22999 | A | II | July 31, 2009 | CADILA HEALTHCARE LTD |
| 24256 | A | II | September 10, 2010 | ALP PHARM BEIJING CO LTD |
| 29051 | A | II | February 20, 2015 | CTX LIFE SCIENCES PVT LTD |
| 29676 | A | II | January 12, 2016 | SIGNA SA DE CV |
| 29998 | A | II | November 6, 2015 | DIVIS LABORATORIES LTD |
| 31570 | A | II | March 31, 2017 | MAITHRI DRUGS PRIVATE LTD |
| 8070 | A | II | May 9, 1989 | PHARMAZELL GMBH 5-AMINOSALICYLIC ACID |
| Parameters | Details |
|---|---|
| Strength | 1.2g |
| Excipients used | sodium carboxymethylcellulose, carnauba wax, stearic acid, silica (colloidal hydrated), sodium starch glycolate (type A), talc, magnesium stearate |
| Composition of coating material | talc, methacrylic acid copolymer type A, methacrylic acid copolymer type B, triethylcitrate, titanium dioxide (E171), red ferric oxide (E172), macrogol 6000 |
| Composition of caspule shell | - |
| Pharmaceutical Development |
The tablet core contains mesalamine with hydrophilic and lipophilic excipients and provides for extended release of mesalamine. The tablet core is a double matrix system made of an inert lipophilic matrix (in which some of the mesalazine is incorporated), and a hydrophilic matrix, that includes a hydrophilic polymer and the remaining mesalazine. The tablet core is coated with a gastro-resistant, pH-dependent polymer film, which only breaks down at pH 6.8 or higher, normally in the terminal ileum. Thus, the Mezavant 1200 mg formulation provides a combination of delayed and extended drug release, thereby aiming for homogenous release of mesalazine throughout the colon. |
| Manufacture of the product | - |
| Tablet / Capsule Image |

|
| Appearance | The red-brown ellipsoidal delayed-release tablet containing 1.2 g mesalamine is debossed on one side with S476. |
| Imprint code / Engraving / Debossment | debossed on one side with S476. |
| Score | no score |
| Color | Red-Brown |
| Shape | OVAL |
| Dimension | 20mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | Shire Inc (US, EU) |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N022000 | 1 | 6773720 | June 8, 2020 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 100 | Acid stage (A): 100 mM HCl Buffer stage (B): Phosphate Buffer, pH 6.4 Buffer stage (C): Phosphate Buffer, pH 7.2 | Acid stage (A): 750 mL; Buffer stage (B): 950 mL; Buffer stage (C): 960 mL | Acid stage (A): 2 hours; Buffer stage (B): 1 hour; Buffer stage (C): 1, 2, 4, 6 and 8 hours | June 10, 2009 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | Mezavant XL | Download |
| UK | Mezavant XL | Download |
| US | LIALDA | Download |
| US | Zydus cadila |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
