| Active Ingredient | LOFEXIDINE HYDROCHLORIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LUCEMYRA | 209229 | US WORLDMEDS LLC | TABLET;ORAL | EQ 0.18MG BASE | EQ 0.18MG BASE | May 16, 2018 | May 16, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
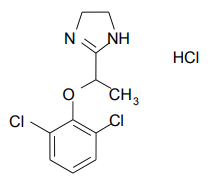 |
| Chemical Name | 2-[1-(2,6dichlorophenoxy)ethyl]-4,5 dihydro-1H- imidazole monohydrochloride |
| CAS No | 21498-08-8 |
| Molecular Formula | C11H12Cl2N2O•HCl |
| Molecular Weight | 295.6 g/mole |
| Appearance | White to off-white crystalline powder |
| Solubility | Freely soluble in methanol, and ethanol. It is slightly soluble in chloroform and practically insoluble in nhexane and benzene. |
| Water Solubility | Freely soluble in water |
| Polymorphism | There are two polymorphic forms of the drug substance |
| pKa (Strongest Acidic) | - |
| pKa (Strongest Basic) | 9.27 |
| Log P | 3.31 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 236oC to 238oC. |
| BCS Class | I |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | LUCEMYRA is a central alpha-2 adrenergic agonist indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults |
| Dosage and Administration |
The usual LUCEMYRA dosage is three 0.18 mg tablets taken orally 4 times daily at 5- to 6-hour intervals LUCEMYRA treatment may be continued for up to14 days with dosing guided by symptoms. Discontinue LUCEMYRA with a gradual dose reduction over 2 to 4 days. Hepatic or Renal Impairment: Dosage adjustments are recommended based on degree of impairment. |
| Mechanism of action | Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic tone. |
| Absorption | LUCEMYRA is well absorbed and achieves peak plasma concentration 3 to 5 hours after administration of a single dose. LUCEMYRA shows approximately dose-proportional pharmacokinetics. Administration of LUCEMYRA with food does not alter its pharmacokinetics.The absolute bioavailability of a single oral LUCEMYRA dose ( 0.36 mg in solution) compared with an intravenous infusion (0.2 mg infused for 200 minutes) was 72%. Mean LUCEMYRA Cmax after the oral dose and intravenous infusion was 0.82 ng/mL (at median Tmax of 3 hours) and 0.64 ng/mL (at median Tmax of 4 hours), respectively. Mean estimates of overall systemic exposure (AUCinf) were 14.9 ng•h/mL and 12.0 ng•h/mL, respectively. |
| Food Effect | Administration of LUCEMYRA with food does not alter its pharmacokinetics. |
| Distribution | Mean LUCEMYRA apparent volume of distribution and volume of distribution values following the administration of an oral dose and an intravenous dose were 480.0 L and 297.9 L, respectively, which are appreciably greater than total body volume, suggesting extensive LUCEMYRA distribution into body tissue.LUCEMYRA protein binding is approximately 55%.LUCEMYRA is not preferentially taken up by blood cells. In a study comparing LUCEMYRA concentrations in plasma and whole blood at the time of peak LUCEMYRA concentrations in human volunteers, it was determined that red blood cells contain approximately 27% the LUCEMYRA concentration of the plasma. |
| Metabolism | From absolute bioavailability results, approximately 30% of the administered LUCEMYRA dose is converted to inactive metabolites during the first pass effect associated with drug absorption from the gut.LUCEMYRA and its major metabolites did not induce or inhibit any CYP450 isoforms, with the exception of a slight inhibition of CYP2D6 by LUCEMYRA, with an IC50 of 4551 nM (approximately 225 times the steady-state Cmax for LUCEMYRA with 0.72 mg 4 times daily dosing). Any LUCEMYRA interaction with CYP2D6 substrates is not expected to be clinically significant.LUCEMYRA is metabolized when incubated in vitro with human liver microsomes, the major contributor to the hepatic metabolism of LUCEMYRA is CYP2D6, with CYP1A2 and CYP2C19 also capable of metabolizing LUCEMYRA. |
| Elimination |
The elimination half-life is approximately 12 hours and mean clearance is 17.6 L/h following an IV infusion. LUCEMYRA has a terminal half-life of approximately 11 to 13 hours following the first dose.At steady-state, the terminal half-life is approximately 17 to 22 hours. Accumulation occurs up to 4 days with repeat dosing, following the recommended dosing regimen. A mass balance study of LUCEMYRA showed nearly complete recovery of radiolabel in urine (93.5%) over 144 hours postdose, with an additional 0.92% recovered in the feces over 216 hours postdose. Thus, it appears that all, or nearly all, of the dose was absorbed, and that the primary route of elimination of the parent drug and its metabolites is via the kidney. Renal elimination of unchanged drug accounts for approximately 15% to 20% of the administered dose. |
| Peak plasma time (Tmax) | 3 to 5 hours |
| Half life | Approximately 12 hours |
| Bioavailability | 72% |
| Age, gender |
Hepatic impairment slows the elimination of LUCEMYRA but exhibits less effect on the peak plasma concentration following a single dose. I |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | EQ 0.18MG BASE |
| Excipients used |
92.6 mg lactose, 12.3 mg citric acid, 1.1 mg povidone, 5.7 mg microcrystalline cellulose, 1.4 mg calcium stearate, 0.7 mg sodium lauryl sulphate |
| Composition of coating material | Opadry OY-S-9480 (contains indigo carmine and sunset yellow). |
| Composition of caspule shell | _ |
| Pharmaceutical Development | Each tablet contains 0.18 lofexidine, equivalent to 0.2 mg of lofexidine hydrochloride, The drug substance polymorphism and particle size distribution are not critical attributes that will affect product performance. |
| Manufacture of the product | Will be updated soon |
| Tablet / Capsule Image |
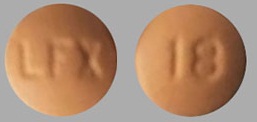
|
| Appearance | Round, convex-shaped, peach colored, film-coated tablets, imprinted with “LFX” on one side and “18” on the other side |
| Imprint code / Engraving / Debossment | Imprinted with “LFX” on one side and “18” on the other side |
| Score | No score |
| Color | Peach |
| Shape | Round |
| Dimension | 7 mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by |
US WorldMeds, LLC 4441 Springdale Road Louisville, KY 4024 |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II | 50 | 0.025 M Potassium phosphate buffer, pH 7.2 | 500 mL | 5, 10, 15,20,30 and 45 min (Q point at 30 min) | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
