| Active Ingredient | LINACLOTIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LINZESS | (NDA) 202811 | FOREST LABS LLC | CAPSULE;ORAL | 145MCG, 290MCG | 290MCG (RS) | August 30, 2012 | August 30, 2017 | - | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
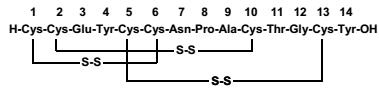 |
| Chemical Name | Linaclotide is a 14-amino acid peptide with the following chemical name: L-cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-Lasparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threonyl-glycyl-L-cysteinyl-L-tyrosine, cyclic (1-6), (2-10), (513)-tris (disulfide). |
| CAS No | 851199-59-2 |
| Molecular Formula | C59H79N15O21S6 |
| Molecular Weight | 1526.8 |
| Appearance | an amorphous, white to off-white powder |
| Solubility | It is slightly soluble in water and aqueous sodium chloride (0.9%). It is sparingly soluble in 0.1 N HCl, slightly soluble in water and very slightly soluble in acetone and acetonitrile. The solubility in aqueous solutions over a pH range of 1.0 to 7.5 is > 100 μg/ml. |
| Water Solubility | 0.701 mg/mL (Predicted) |
| Polymorphism | Linaclotide is an amorphous peptide, no crystalline material has been observed in the x-ray powder diffractogram. |
| pKa (Strongest Acidic) | 3.06 (Predicted) |
| pKa (Strongest Basic) | 7.65 (Predicted) |
| Log P | - |
| Identification | - |
| Degradation | - |
| Hygroscopic | hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | III |
| Manufacture of API | Linaclotide is manufactured by a five step process.Linaclotide is supplied by two active substance manufacturers. Both suppliers provided adequate information on the active substance development, manufacturing process, control of starting materials, reagents and solvents, and control of critical steps and intermediates in the form of an active substance master file (ASMF). |
| Parameters | Details |
|---|---|
| Indications and Usage | Irritable Bowel Syndrome with Constipation (IBS-C) : LINZESS (linaclotide) is indicated in adults for the treatment of irritable bowel syndrome with constipation (IBS-C). Chronic Idiopathic Constipation (CIC) : LINZESS is indicated in adults for the treatment of chronic idiopathic constipation (CIC). |
| Dosage and Administration |
Irritable Bowel Syndrome with Constipation (IBS-C): The recommended dose of LINZESS is 290 mcg taken orally once daily on an empty stomach, at least 30 minutes prior to the first meal of the day. Chronic Idiopathic Constipation (CIC): The recommended dose of LINZESS is 145 mcg taken orally once daily on an empty stomach, at least 30 minutes prior to the first meal of the day. |
| Mechanism of action | Linaclotide is a guanylate cyclase-C (GC-C) agonist. Both linaclotide and its active metabolite bind to GCC and act locally on the luminal surface of the intestinal epithelium. Activation of GC-C results in an increase in both intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP). Elevation in intracellular cGMP stimulates secretion of chloride and bicarbonate into the intestinal lumen, mainly through activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit. In animal models, linaclotide has been shown to both accelerate GI transit and reduce intestinal pain. The linaclotideinduced reduction in visceral pain in animals is thought to be mediated by increased extracellular cGMP, which was shown to decrease the activity of pain-sensing nerves. |
| Absorption | LINZESS is minimally absorbed with low systemic availability following oral administration. Concentrations of linaclotide and its active metabolite in plasma are below the limit of quantitation after oral doses of 145 mcg or 290 mcg were administered. Therefore, standard pharmacokinetic parameters such as area under the curve (AUC), maximum concentration (Cmax), and half-life (t½) cannot be calculated. |
| Food Effect | In a cross-over study, 18 healthy subjects were given LINZESS 290 mcg for 7 days both in the non-fed and fed state. Neither linaclotide nor its active metabolite was detected in the plasma. Taking LINZESS immediately after the high fat breakfast resulted in looser stools and a higher stool frequency compared with taking it in the fasted state. In clinical trials, LINZESS was administered on an empty stomach, at least 30 minutes before breakfast. |
| Distribution | Given that linaclotide plasma concentrations following therapeutic oral doses are not measurable, linaclotide is expected to be minimally distributed to tissues. |
| Metabolism | Linaclotide is metabolized within the gastrointestinal tract to its principal, active metabolite by loss of the terminal tyrosine moiety. Both linaclotide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids. |
| Elimination | Active peptide recovery in the stool samples of fed and fasted subjects following the daily administration of 290 mcg of LINZESS for seven days averaged about 5% (fasted) and about 3% (fed) and virtually all as the active metabolite. |
| Peak plasma time (Tmax) | - |
| Half life | - |
| Bioavailability | - |
| Age, gender | Clinical studies to determine the impact of age and gender on the pharmacokinetics of LINZESS have not been conducted. See Use in Specific Populations (8.5) for information regarding patients aged 65 years and older. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 24992 | A | II | May 27, 2011 | CORDEN PHARMA COLORADO INC |
| 25021 | A | II | June 1, 2011 | POLYPEPTIDE LABORATORIES SWEDEN AB |
| 25025 | A | II | June 7, 2011 | POLYPEPTIDE LABORATORIES INC |
| 28273 | A | II | May 5, 2014 | BACHEM AMERICAS INC |
| 29708 | A | II | Septmber 22, 2015 | AURO PEPTIDES LTD |
| 30266 | A | II | November 16, 2016 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 30274 | A | II | March 10, 2016 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 30421 | A | II | March 29, 2016 | MSN LIFE SCIENCES PRIVATE LTD |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 145MCG | 290MCG | |
| Excipients used | calcium chloride dihydrate (0.51MG), L-leucine (0.73MG), hypromellose (0.5MG), microcrystalline cellulose (97.615MG), gelatin, and titanium dioxide | calcium chloride dihydrate (1.02MG), L-leucine (1.46MG), hypromellose (1MG), microcrystalline cellulose (195.23MG), gelatin, and titanium dioxide | |
| Composition of coating material | - | ||
| Composition of caspule shell |
Capsule shell: red iron oxide (E172), titanium dioxide (E171), yellow iron oxide (E172) and gelatin. Capsule ink: shellac, propylene glycol, concentrated ammonia solution, potassium hydroxide, titanium dioxide (E171) and black iron oxide (E172). |
||
| Pharmaceutical Development |
In view of the low doses of linaclotide, the applicant selected gelatin capsules filled with linaclotide-coated microcrystalline cellulose beads to develop an immediate release formulation. Linaclotide was classified as a class III active substance per BCS. By selecting a process where linaclotide is dissolved and sprayed onto microcrystalline cellulose beads, the particle size distribution is not considered a critical quality attribute for the dissolution behaviour. The list of excipients include: microcrystalline cellulose (diluent), hypromellose (binding agent), hydrochloric acid (acidifying agent), calcium chloride dihydrate and leucine (stabilising agents). The gelatin capsule consists of gelatin (shell matrix), titanium dioxide (opacifer), red iron oxide and yellow iron oxide (colourants). The list of excipients include: microcrystalline cellulose (diluent), hypromellose (binding agent), hydrochloric acid (acidifying agent), calcium chloride dihydrate and leucine (stabilising agents). The gelatin capsule consists of gelatin (shell matrix), titanium dioxide (opacifer), red iron oxide and yellow iron oxide (colourants). |
||
| Manufacture of the product | The manufacturing process consists of three main steps: i) dissolution of the active substance and the excipients in an acidic coating solution, ii) coating of this solution onto inert microcrystalline cellulose beads and iii) encapsulation of the appropriate amount of coated beads in hard gelatin capsules. | ||
| Tablet / Capsule Image |

|

|
|
| Appearance | white to off-white opaque with gray imprint “FL 145” | white to off-white opaque with gray imprint “FL 290” | |
| Imprint code / Engraving / Debossment | gray imprint “FL 145” | gray imprint “FL 290” | |
| Score | no score | no score | |
| Color | white to off-white opaque | white to off-white opaque | |
| Shape | Capsule | Capsule | |
| Dimension | 16mm | 18mm | |
| Mfg by | - | ||
| Mfg for | - | ||
| Marketed by | Forest Pharmaceuticals, Inc. (US) | ||
| Distributed by | Forest Pharmaceuticals, Inc. (US) | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N202811 | 1 | 7304036 | August 30, 2026 | Y | Y | U - 1278 | - | Download |
| N202811 | 1 | 8110553 | January 28, 2024 | - | - | U - 1278 | - | Download |
| N202811 | 1 | 8748573 | June 20, 2031 | - | - | U - 1515 | - | Download |
| N202811 | 1 | 8802628 | July 24, 2031 | - | Y | - | - | Download |
| N202811 | 1 | 8933030 | February 17, 2031 | - | Y | - | - | Download |
| N202811 | 1 | 7371727 | January 28, 2024 | Y | - | - | - | Download |
| N202811 | 1 | 7704947 | January 28, 2024 | Y | Y | - | - | Download |
| N202811 | 1 | 7745409 | January 28, 2024 | Y | Y | - | - | Download |
| N202811 | 1 | 8080526 | January 28, 2024 | Y | Y | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| I (Basket) | 50 | 50 mM Phosphate Buffer, pH 4.5 | 500 | 5, 10, 15, 20 and 30 | December 24, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | CONSTELLA | Download |
| UK | CONSTELLA | Download |
| US | LINZESS | Download |
| Only Constella 290MG capsule is approved in EU. Exclusivity Code: Exclusivity Expiration; New Strength; Jan 25, 2020 |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
