| Active Ingredient | LENALIDOMIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REVLIMID | (NDA) 021880 | CELGENE | CAPSULE;ORAL | 2.5 MG, 5 MG, 10 MG, 15 MG, 20 MG, 25 MG | 20 MG | December 27, 2005 | _ | Feb 17, 2022 | 1 New molecular entity (NME) | P Priority review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
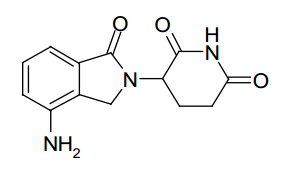 |
| Chemical Name | 3(4-amino-1-oxo 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione |
| CAS No | 191732-72-6 |
| Molecular Formula | C13H13N3O3 |
| Molecular Weight | 259.3 |
| Appearance | Off-white to pale-yellow solid powder |
| Solubility | Soluble in organic solvent/water mixtures, and buffered aqueous solvents. More soluble in organic solvents and low pH solutions. Solubility was significantly lower in less acidic buffers, ranging from about 0.4 to 0.5 mg/ml |
| Water Solubility | 2.33 mg/mL (Predicted\0 |
| Polymorphism | It exhibits the phenomenon of polymorphism but the commercial process leads only to the desired polymorph. |
| pKa (Strongest Acidic) | 11.61 |
| pKa (Strongest Basic) | 2.31 |
| Log P | -0.4 |
| Identification | IR and X-ray diffraction |
| Degradation | Stable |
| Hygroscopic | Non-hygroscopic |
| Photostability study | Not light sensitive |
| Melting Point | 269-271°C |
| BCS Class | III |
| Manufacture of API | Synthetic process consists of 4 steps starting from alpha-aminoglutarimide hydrochloride and methyl 2-bromomethyl-3-nitrobenzoate. The process can be summarised as follow: coupling step, reduction step (crude lenalidomide), recrystallisation (pre-micronised lenalidomide) and micronisation. |
| Parameters | Details |
|---|---|
| Indications and Usage | REVLIMID is a thalidomide analogue indicated for the treatment of patients with: • Multiple myeloma (MM), in combination with dexamethasone. • Transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities. • Mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib. Limitations of Use: • REVLIMID is not indicated and is not recommended for the treatment of patients with chronic lymphocytic leukemia (CLL) outside of controlled clinical trials |
| Dosage and Administration |
• MM: 25 mg once daily orally on Days 1-21 of repeated 28-day cycles. • MDS: 10 mg once daily. • MCL: 25 mg once daily orally on Days 1-21 of repeated 28-day cycles. • Continue or modify dosing based on clinical and laboratory findings. • Renal impairment: Adjust starting dose in patients with moderate or severe renal impairment and on dialysis (CLcr<60 mL/min) |
| Mechanism of action | Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma. Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis. |
| Absorption | Lenalidomide is rapidly absorbed following oral administration. Following single and multiple doses of REVLIMID in patients with MM or MDS the maximum plasma concentrations occurred between 0.5 and 6 hours post-dose. The single and multiple dose pharmacokinetic disposition of lenalidomide is linear with AUC and Cmax values increasing proportionally with dose. Multiple dosing at the recommended dose-regimen does not result in drug accumulation. Systemic exposure (AUC) of lenalidomide in MM and MDS patients with normal or mild renal function (CLcr ≥ 60 mL/min) is approximately 60% higher as compared to young healthy male subjects. Population pharmacokinetic analyses show that the oral absorption rate of lenalidomide in patients with MCL is similar to that observed in patients with MM or MDS. |
| Food Effect | Administration of a single 25 mg dose of REVLIMID with a high-fat meal in healthy subjects reduces the extent of absorption, with an approximate 20% decrease in AUC and 50% decrease in Cmax. In the trials where the efficacy and safety were established for REVLIMID, the drug was administered without regard to food intake. REVLIMID can be administered with or without food. |
| Distribution | In vitro (14C)-lenalidomide binding to plasma proteins is approximately 30%. Lenalidomide is present in semen at 2 hours (1379 ng/ejaculate) and 24 hours (35 ng/ejaculate) after the administration of REVLIMID 25 mg daily. |
| Metabolism | Lenalidomide -undergoes limited metabolism. Unchanged lenalidomide is the predominant circulating component in humans. Two identified metabolites are 5-hydroxy-lenalidomide and N-acetyl-lenalidomide; each constitutes less than 5% of parent levels in circulation. |
| Elimination | Elimination is primarily renal. Following a single oral administration of [14C]-lenalidomide (25 mg) to healthy subjects, approximately 90% and 4% of the radioactive dose is eliminated within ten days in urine and feces, respectively. Approximately 82% of the radioactive dose is excreted as lenalidomide in the urine within 24 hours. Hydroxy-lenalidomide and N-acetyl-lenalidomide represent 4.59% and 1.83% of the excreted dose, respectively. The renal clearance of lenalidomide exceeds the glomerular filtration rate. The mean half-life of lenalidomide is 3 hours in healthy subjects and 3 to 5 hours in patients with MM, MDS or MCL. |
| Peak plasma time (Tmax) | 0.5 and 6 hours post-dose |
| Half life | 3 hours in healthy subjects and 3 to 5 hours in patients with MM, MDS or MCL |
| Bioavailability | - |
| Age, gender | No dedicated clinical studies have been conducted to evaluate pharmacokinetics of lenalidomide in the elderly. Population pharmacokinetic analyses included patients with ages ranging from 39 to 85 years old and show that age does not influence the disposition of lenalidomide. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 23410 | A | II | December 29, 2009 | MYLAN LABORATORIES LTD |
| 24264 | A | II | December 2, 2010 | APICORE US LLC |
| 25872 | I | II | March 30, 2012 | DR REDDYS LABORATORIES LTD |
| 27116 | A | II | June 4, 2013 | FIS FABBRICA ITALIANA SINTETICI SPA |
| 27905 | A | II | February 3, 2014 | RELIANCE LIFE SCIENCES PVT LT |
| 29690 | A | II | September 30, 2015 | DR REDDYS LABORATORIES LTD (DIMETHYLFORMAMIDE SOLVATE) |
| 29691 | A | II | September 30, 2015 | DR REDDYS LABORATORIES LTD (POVIDONE PREMIX) |
| Parameters | Details | ||||||
|---|---|---|---|---|---|---|---|
| Strength | 2.5 MG | 5 MG | 10 MG | 15 MG | 20 MG | 25 MG | |
| Excipients used | Lactose anhydrous (73.5 mg), microcrystalline cellulose, croscarmellose sodium, and magnesium stearate | Lactose anhydrous ( 147 mg ), microcrystalline cellulose (40 mg), croscarmellose sodium (6 mg), and magnesium stearate (2 mg) | Lactose anhydrous (294 mg), microcrystalline cellulose (80 mg), croscarmellose sodium (12 mg), and magnesium stearate (4 mg) | Lactose anhydrous (289 mg), microcrystalline cellulose (80 mg), croscarmellose sodium (12 mg), and magnesium stearate (4 mg) | Lactose anhydrous (244.5 mg), microcrystalline cellulose, croscarmellose sodium, and magnesium stearate | Lactose anhydrous (200 mg), microcrystalline cellulose (159 mg), croscarmellose sodium (12 mg), and magnesium stearate (4 mg) | |
| Composition of coating material | - | ||||||
| Composition of caspule shell | Gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink | Gelatin, titanium dioxide and black ink | Gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink |
Gelatin, FD&C blue #2, titanium dioxide and black ink |
Gelatin, FD&C blue #2, yellow iron oxide, titanium dioxide and black ink | Gelatin, titanium dioxide and black ink | |
| Pharmaceutical Development |
Active Substance: The pharmaceutical development has taken into consideration the main aspects of the active substance: particle size and polymorphic form. Formulation Development: A hard capsule dosage form was chosen due to its favourable performance in manufacturing studies and analytical testing. Revlimid capsules, 5 and 10 mg, are dose proportional and use a common blend whereas the 15 and 25 mg are slightly different for the ingredients ratio. Formulation development is sufficiently detailed. Micronisation has been performed to ensure blend and content uniformity of the active substance present at a low level in the medicinal product formulation. Overages: No overage is included in the formulation for Revlimid capsules 5, 10, 15 and 25 mg. Physicochemical and Biological Properties: The impact of physicochemical properties (particle size and polymorphic form) of lenalidomide that could theoretically affect the performance of the medicinal product has been studied. It can be concluded that neither the particle size (when included in the distribution range of micronised particles), neither does the polymorphic form affect the dissolution of the capsules. Manufacturing Process Development: The manufacturing process is a simple blending and filling process. The manufacturing development has been adequately described. |
||||||
| Manufacture of the product | The manufacturing process is simple and consists ofdry blending of the ingredients and filling the capsules. It has been adequately described including the equipment and the operating parameters. Appropriate in-process controls of the critical steps and intermediates have been set, such as control of the fill weight of the capsules. | ||||||
| Tablet / Capsule Image |
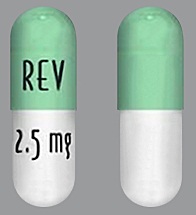
|
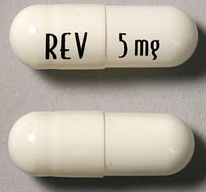
|
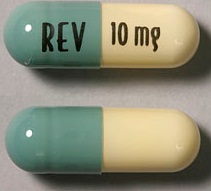
|
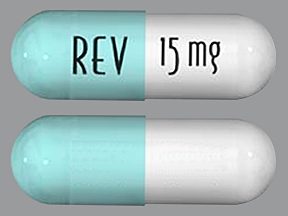
|

|
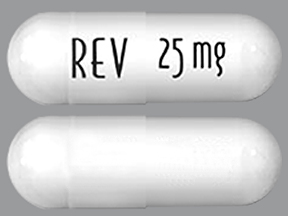
|
|
| Appearance | White and blue-green opaque hard capsules imprinted “REV” on one half and “2.5 mg” on the other half in black ink | White opaque capsules imprinted “REV” on one half and “5 mg” on the other half in black ink | Blue/green and pale yellow opaque capsules imprinted “REV” on one half and “10 mg” on the other half in black ink | Powder blue and white opaque capsules imprinted “REV” on one half and “15 mg” on the other half in black ink | Powder blue and blue-green opaque hard capsules imprinted “REV” on one half and “20 mg” on the other half in black ink | White opaque capsules imprinted “REV” on one half and “25 mg” on the other half in black ink | |
| Imprint code / Engraving / Debossment | Imprinted “REV” on one half and “2.5 mg” on the other half in black ink | Imprinted “REV” on one half and “5 mg” on the other half in black ink | Imprinted “REV” on one half and “10 mg” on the other half in black ink | Imprinted “REV” on one half and “15 mg” on the other half in black ink | Imprinted “REV” on one half and “20 mg” on the other half in black ink | Imprinted “REV” on one half and “25 mg” on the other half in black ink | |
| Score | No Score | No Score | No Score | No Score | No Score | No Score | |
| Color | White and blue-green | White opaque capsules | Blue/green and pale yellow | Powder blue and white | Powder blue and blue-green | White | |
| Shape | Capsules | Capsules | Capsules | Capsules | Capsules | Capsules | |
| Dimension | Size 4, 14.3 mm | Size 2, 18.0 mm | Size 0, 21.7 mm | Size 0, 21.7 mm | Size 0, 21.7 mm | Size 0, 21.7 mm | |
| Mfg by |
Penn Pharmaceutical Services Limited (EU/UK) Celgene Europe Limited (EU/UK) |
||||||
| Mfg for | Celgene Corporation (US) | ||||||
| Marketed by | Celgene Europe Limited (EU/UK) | ||||||
| Distributed by | - | ||||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021880 | 1 | 5635517 | October 4, 2019 | Y | - | U - 1211 | - | Download |
| N021880 | 1 | 6045501 | August 28, 2018 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 6281230 | July 24, 2016 | - | - | U - 1212 | - | Download |
| N021880 | 1 | 6281230 | July 24, 2016 | - | - | U - 1212 | - | Download |
| N021880 | 1 | 6315720 | October 23, 2020 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 6555554 | July 24, 2016 | - | Y | U - 1211 | - | Download |
| N021880 | 1 | 6561976 | August 28, 2018 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 6561977 | October 23, 2020 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 6755784 | October 23, 2020 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 6908432 | August 28, 2018 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 7119106 | July 24, 2016 | - | Y | - | - | Download |
| N021880 | 1 | 7189740 | April 11, 2023 | - | - | U - 1215 | - | Download |
| N021880 | 1 | 7465800 | April 27, 2027 | Y | Y | - | - | Download |
| N021880 | 1 | 7468363 | October 7, 2023 | - | - | U - 1414 | - | Download |
| N021880 | 1 | 7855217 | November 24, 2024 | Y | Y | - | - | Download |
| N021880 | 1 | 7968569 | October 7, 2023 | - | - | U - 1216 | - | Download |
| N021880 | 1 | 8204763 | August 28, 2018 | - | - | U - 1249 | - | Download |
| N021880 | 1 | 8288415 | July 24, 2016 | Y | Y | - | - | Download |
| N021880 | 1 | 8315886 | October 23, 2020 | - | - | U - 1249 | - | Download |
| N021880 | 1 | 8404717 | April 11, 2023 | - | - | U - 1215 | - | Download |
| N021880 | 1 | 8530498 | May 15, 2023 | - | - | U - 1216 | - | Download |
| N021880 | 1 | 8589188 | August 28, 2018 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 8626531 | October 23, 2020 | - | - | U - 1210 | - | Download |
| N021880 | 1 | 8648095 | May 15, 2023 | - | - | U - 1216 | - | Download |
| N021880 | 1 | 8741929 | March 8, 2028 | - | - | U - 1414 | - | Download |
| N021880 | 1 | 9056120 | April 11, 2023 | - | - | U - 1215 | - | Download |
| N021880 | 1 | 9101621 | May 15, 2023 | - | - | U - 1216 | - | Download |
| N021880 | 1 | 9101622 | May 15, 2023 | - | - | U - 1216 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.01 N HCl | 900 | 10, 15, 20, 30 and 45 | April 15, 2008 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| Australia | REVLIMID (Celgene Pty Limited) | |
| Canada | REVLIMID | |
| EU | BLUEPHARMA - INDUSTRIA FARMACEUTICA, S.A (Generic, Pipeline Under Consideration) | |
| EU | Grindeks AS (Generic, Under Development / Product with vertical integration ) | |
| EU | REVLIMID | Download |
| Inida | Accure Labs Pvt. Ltd.(Generic) | |
| Inida | Flagship Biotech International Pvt. Ltd (Generic) | |
| Inida | Nishchay Pharmaceuticals Pvt. Ltd.(Nalmide 5, Patented) | |
| Portugal | TECNIMEDE.(Dossier Status- 4th Quarter 2017) | |
| South Africa | REVLIMID (Key Oncologics (Pty) Ltd) | |
| UK | REVLIMID | Download |
| US | REVLIMID | Download |
| Lenalidomide has an asymmetric carbon atom and can exist as the optically active forms S(-) and R(+), and is produced as a racemic mixture with a net optical rotation of zero. It is a synthetic derivative of glutamic acid and is structurally close to thalidomide (identical backbone but differs from thalidomide by removing an oxygen from the phthalyl ring and by adding an amine group). Although it is chiral and possesses an asymmetric carbon, it has been developed as a racemic mixture since it undergoes racemisation under physiological conditions. It is obtained as a hemihydrate form and is non-hygroscopic. Revlimid 7.5 mg hard capsules is available in EU only. I - 672 Exclusivity Expiration on Jun 5, 2016. I - 706 Exclusivity Expiration on Feb 17, 2018. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
