| Active Ingredient | ETRAVIRINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTELENCE | (NDA) 022187 | JANSSEN R AND D | TABLET;ORAL | 25MG, 100MG, 200MG | 25MG, 100MG, 200MG (RS) | January 18, 2008 | - | - | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
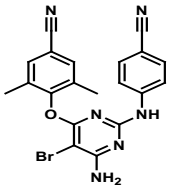 |
| Chemical Name | 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5dimethylbenzonitrile |
| CAS No | 269055-15-4 |
| Molecular Formula | C20H15BrN6O |
| Molecular Weight | 435.28 |
| Appearance | a white to slightly yellowish brown powder |
| Solubility | Etravirine is practically insoluble in water over a wide pH range. It is very slightly soluble in propylene glycol and slightly soluble in ethanol. Etravirine is soluble in polyethylene glycol (PEG)400 and freely soluble in some organic solvents (e.g., N,N-dimethylformamide and tetrahydrofuran). |
| Water Solubility | 0.0169 mg/mL (Predicted) |
| Polymorphism | Etravirine may exist in two highly crystalline polymorphs. During production one polymorph has been consistently produced and the crystallization of this polymorph is under control. The polymorphism is not considered as critical for the efficacy of the product. |
| pKa (Strongest Acidic) | 12.49 (Predicted) |
| pKa (Strongest Basic) | 4.13 (Predicted) |
| Log P | 3.67 (Predicted) |
| Identification | HPLC and IR spectra |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | IV |
| Manufacture of API | Etravirine is manufactured from two starting materials by a three steps process. A purification is performed at each step. The three steps have an impact on critical quality attributes of the final etravirine drug substance and thus, are critical steps of the synthesis. |
| Parameters | Details |
|---|---|
| Indications and Usage | INTELENCE, in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced patients ages 6 years and older, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents. The indication for adult use is based on Week 48 analyses from 2 randomized, double-blind, placebo-controlled trials of INTELENCE. Both studies were conducted in clinically advanced, 3-class antiretroviral (NNRTI, N[t]RTI, PI) treatment-experienced adults. The indication for pediatric use is based on 24-week analyses of a single-arm, Phase 2 trial in antiretroviral treatment-experienced pediatric subjects 6 years to less than 18 years of age. In treatment-experienced adult and pediatric patients, the following points should be considered when initiating therapy with INTELENCE: • Treatment history and resistance testing should guide the use of INTELENCE due to concerns for potential cross-resistance. • In patients who have experienced virologic failure on an NNRTI-containing regimen, do not use INTELENCE in combination with only N[t]RTIs. • The use of other active antiretroviral agents with INTELENCE is associated with an increased likelihood of treatment response. • The safety and efficacy of INTELENCE have not been established in pediatric patients less than 6 years of age or in treatment-naïve adult or pediatric patients. |
| Dosage and Administration |
Adult Patients: The recommended oral dose of INTELENCE tablets is 200 mg (one 200 mg tablet or two 100 mg tablets) taken twice daily following a meal [see Clinical Pharmacology (12.3)]. The type of food does not affect the exposure to etravirine. Pediatric Patients (6 years to less than 18 years of age): The recommended dose of INTELENCE® for pediatric patients 6 years to less than 18 years of age and weighing at least 16 kg is based on body weight not exceeding the recommended adult dose. INTELENCE tablet(s) should be taken orally, following a meal. The type of food does not affect the exposure to etravirine. The safety and efficacy of INTELENCE have not been established in children less than 6 years of age. Healthcare professionals should pay special attention to the accurate dose selection of INTELENCE, the transcription of the medication order, the dispensing information and the dosing instructions to minimize the risk of medication errors, overdosing, and underdosing. |
| Mechanism of action | INTELENCE (etravirine) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus type 1 (HIV-1). Etravirine is an NNRTI of human immunodeficiency virus type 1 (HIV-1). Etravirine binds directly to reverse transcriptase (RT) and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. Etravirine does not inhibit the human DNA polymerases α, β, and γ. |
| Absorption |
Following oral administration, etravirine was absorbed with a Tmax of about 2.5 to 4 hours. The absolute oral bioavailability of INTELENCE is unknown. In healthy subjects, the absorption of etravirine is not affected by co-administration of oral ranitidine or omeprazole, drugs that increase gastric pH. |
| Food Effect | The systemic exposure (AUC) to etravirine was decreased by about 50% when INTELENCE was administered under fasting conditions, as compared to when INTELENCE was administered following a meal. Therefore, INTELENCE should always be taken following a meal. Within the range of meals studied, the systemic exposures to etravirine were similar. The total caloric content of the various meals evaluated ranged from 345 kilocalories (17 grams fat) to 1160 kilocalories (70 grams fat). |
| Distribution | Etravirine is about 99.9% bound to plasma proteins, primarily to albumin (99.6%) and alpha 1-acid glycoprotein (97.66% to 99.02%) in vitro. The distribution of etravirine into compartments other than plasma (e.g., cerebrospinal fluid, genital tract secretions) has not been evaluated in humans. |
| Metabolism | In vitro experiments with human liver microsomes (HLMs) indicate that etravirine primarily undergoes metabolism by CYP3A, CYP2C9, and CYP2C19 enzymes. The major metabolites, formed by methyl hydroxylation of the dimethylbenzonitrile moiety, were at least 90% less active than etravirine against wild-type HIV in cell culture. |
| Elimination | After single dose oral administration of 800 mg 14C-etravirine, 93.7% and 1.2% of the administered dose of 14Cetravirine was recovered in the feces and urine, respectively. Unchanged etravirine accounted for 81.2% to 86.4% of the administered dose in feces. Unchanged etravirine was not detected in urine. The mean (± standard deviation) terminal elimination half-life of etravirine was about 41 (± 20) hours. |
| Peak plasma time (Tmax) | 2.5 to 4 hours |
| Half life | 41 (± 20) hours |
| Bioavailability | - |
| Age, gender |
No significant pharmacokinetic differences have been observed between males and females. Population pharmacokinetic analysis of etravirine in HIV-infected subjects did not show an effect of race on exposure to etravirine. Population pharmacokinetic analysis in HIV-infected subjects showed that etravirine pharmacokinetics are not considerably different within the age range (18 to 77 years) evaluated. The pharmacokinetics of etravirine in 101 treatment-experienced HIV-1-infected pediatric subjects, 6 years to less than 18 years of age and weighing at least 16 kg showed that the administered weight-based dosages (approximately 5.2 mg per kg twice daily up to the adult recommended doses) resulted in etravirine exposure comparable to that in adults receiving INTELENCE 200 mg twice daily when administered at a dose corresponding to 5.2 mg per kg twice daily. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 20440 | A | II | April 18, 2007 | JANSSEN PHARMACEUTICA NV |
| 25550 | A | II | December 1, 2011 | HETERO LABS LTD |
| 27923 | A | II | March 7, 2014 | APOTEX PHARMACHEM INDIA PVT LTD |
| 28813 | A | II | December 22, 2014 | MYLAN LABORATORIES LTD |
| 29404 | A | II | May 31, 2015 | MSN LIFE SCIENCES PRIVATE LTD |
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 25MG | 100MG | 200MG | |
| Excipients used | hypromellose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and lactose monohydrate | hypromellose (300MG), microcrystalline cellulose (184.4MG), colloidal silicon dioxide (1.6MG), croscarmellose sodium (40MG), magnesium stearate (4MG) and lactose monohydrate (160MG) | hypromellose (600MG), silicified microcrystalline cellulose (450.2MG), microcrystalline cellulose (70MG), colloidal silicon dioxide (2.8MG), croscarmellose sodium (70MG) and magnesium stearate (7MG) | |
| Composition of coating material | - | |||
| Composition of caspule shell | - | |||
| Pharmaceutical Development | As etravirine is a compound with low aqueous solubility, the manufacturer improved the aqueous solubility, and as such also the bioavailability, by modifying the physical state of the drug (to the amorphous state) by means of the solid dispersion technology. During development of the formulation, several manufacturing techniques have been compared in order to improve the bioavailability and reduce tablet size at the same time. Clinical trials have shown that better bioavailability was obtained by using tablets manufactured from spray-dried material. Therefore, spray drying was selected as the preferred manufacturing technique of etravirine solid dispersions. In addition, formulation studies have been performed to identify an adequate stabilizer for the active substance in solid dispersions. | |||
| Manufacture of the product | The manufacturing process starts with a spray-drying process in order to form the amorphous state of etravirine. After spray drying and mixing the spray-dried powder with various inactive ingredients, which are typical for an immediate release tablet formulation, the final solid dosage form was obtained through compaction and compression. | |||
| Tablet / Capsule Image |
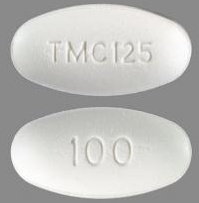
|

|
||
| Appearance | white to off-white, oval, scored tablets debossed with “TMC” on one side | white to off-white oval tablets debossed with “TMC125” on one side and “100” on the other side | white to off-white, biconvex, oblong tablets debossed with “T200” on one side | |
| Imprint code / Engraving / Debossment | debossed with “TMC” on one side and scored on reverse side | debossed with “TMC125” on one side and “100” on the other side | debossed with “T200” on one side and plain on reverse side | |
| Score | score | no score | no score | |
| Color | white to off-white | white to off-white | white to off-white | |
| Shape | Oval | Oval | Oblong | |
| Dimension | - | 19mm | 22mm | |
| Mfg by | Janssen Cilag (US, EU) | |||
| Mfg for | Janssen Cilag (US) | |||
| Marketed by | Janssen Cilag (EU) | |||
| Distributed by | - | |||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N022187 | 1 | 6878717 | November 5, 2019 | - | - | U - 1237 | - | Download |
| N022187 | 1 | 7037917 | December 13, 2020 | Y | Y | U - 1237 | - | Download |
| N022187 | 1 | 7887845 | March 25, 2019 | - | Y | - | - | Download |
| N022187 | 1 | 8003789 | November 1, 2019 | Y | Y | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 1.0 % Sodium lauryl sulfate (SLS) in 0.01 M HCl in two phases: Phase 1: 500 mL of degassed 0.01 M HCl for 10 minutes. Phase 2: Add 400 mL of 2.25% SLS in 0.01 M HCl. | 500 (phase 1): 900 (phase 2) | Phase 1: No Sampling. Phase 2: 5, 10, 20, 30, 45, 60 and 90 | August 14, 2014 [For Etravirine (25 and 100 mg)] |
| II (Paddle) | 70 | 1.0 % Sodium lauryl sulfate (SLS) in 0.01 M HCl in two phases: Phase 1: 1000 mL of degassed 0.01 M HCl for 10 minutes. Phase 2: Add 800 mL of 2.25% SLS in 0.01 M HCl. | 1000 (phase 1): 1800 (phase 2) | Phase 1: No Sampling. Phase 2: 5, 10, 20, 30, 45, 60 and 90 | June 30, 2011 [For Etravirine (200 mg)] |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | INTELENCE | Download |
| UK | INTELENCE (200MG) | Download |
| US | INTELENCE | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
