| Active Ingredient | ELAGOLIX SODIUM |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORILISSA | 210450 | ABBVIE INC | TABLET;ORAL | EQ 150MG BASE, EQ 200MG BASE | EQ 200MG BASE | July 23, 2018 | July 23, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
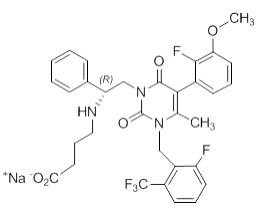 |
| Chemical Name | sodium 4-({(1R)-2-[5-(2-fluoro-3methoxyphenyl)-3-{[2-fluoro-6-(trifluoromethyl)phenyl]methyl}-4-methyl-2,6-dioxo-3,6dihydropyrimidin-1(2H)-yl]-1-phenylethyl}amino)butanoate |
| CAS No | 832720-36-2 |
| Molecular Formula | C32H29F5N3O5Na |
| Molecular Weight | 653.58 g/mole (sodium salt), 631.60 g/mol (free form) |
| Appearance | White to off white to light yellow powder |
| Solubility | highly soluble per the biopharmaceutics classification system (BCS) throughout the physiological pH range. The lowest solubility at pH 5.65 is 0.890 mg/mL. Therefore, the solubility is relative high (200 mg/ 0.890 mg/mL= 220 mL < 240 mL). pH 5 buffer solution : (> 250 g/L) pH 7 buffer solution: (> 250 g/L) pH 9 buffer solution: (> 250 g/L) |
| Water Solubility | Freely soluble in water (> 250 g/L) |
| Polymorphism | Drug substance is amorphous and there is no crystalline form identified |
| pKa (Strongest Acidic) | 4.0 and 7.9 |
| pKa (Strongest Basic) | 9.04 |
| Log P | pH 4: 1.8 pH 7: 1.8 pH 9: 0.7 |
| Identification | - |
| Degradation | - |
| Hygroscopic | Hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | III |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | ORILISSA is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the management of moderate to severe pain associated with endometriosis. |
| Dosage and Administration |
Normal liver function or mild hepatic impairment: 150 mg once daily for up to 24 months or 200 mg twice daily for up to 6 months. Moderate hepatic impairment: 150 mg once daily for up to 6 months. |
| Mechanism of action | ORILISSA is a GnRH receptor antagonist that inhibits endogenous GnRH signaling by binding competitively to GnRH receptors in the pituitary gland. Administration of ORILISSA results in dose-dependent suppression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to decreased blood concentrations of the ovarian sex hormones, estradiol and progesterone. |
| Absorption | Effect of high-fat meal (relative to fasting): AUC: ↓24%, Cmax: ↓36% |
| Food Effect | - |
| Distribution |
% Bound to human plasma proteins : 80 Blood-to-plasma ratio : 0.6 |
| Metabolism | CYP3A (major) Minor pathways include: CYP2D6, CYP2C8, and uridine glucuronosyl transferases (UGTs) |
| Elimination |
Major route of elimination: Hepatic metabolism % of dose excreted in urine: <3 % of dose excreted in feces: 90 |
| Peak plasma time (Tmax) | 1.0 hours |
| Half life | 4-6 hours |
| Bioavailability | - |
| Age, gender | Elagolix exposures (Cmax and AUC) are not altered by renal impairment. The mean exposures are similar for women with moderate to severe or end stage renal disease (including women on dialysis) compared to women with normal renal function. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||
|---|---|---|---|
| Strength | 150 MG | 200 MG | |
| Excipients used | Mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate (Coated tablet weight: 468 mg) |
mannitol, sodium carbonate monohydrate, pregelatinized starch, povidone, magnesium stearate (Coated tablet weight: 624 mg) |
|
| Composition of coating material | Polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and carmine high tint | polyvinyl alcohol titanium dioxide, polyethylene glycol, talc, and iron oxide red. | |
| Composition of caspule shell | NA | ||
| Pharmaceutical Development |
Each tablet contains 155.2 mg of elagolix sodium (equivalent to 150 mg of elagolix) as the active ingredient. Each tablet contains 207.0 mg of elagolix sodium (equivalent to 200 mg of elagolix) as the active ingredient |
||
| Manufacture of the product | Updated soon.. | ||
| Tablet / Capsule Image |

|

|
|
| Appearance | Light pink, oblong, film-coated tablets with “EL 150” debossed on one side | Light orange, oblong, film-coated tablets with “EL 200” debossed on one side | |
| Imprint code / Engraving / Debossment | “EL 150” debossed on one side | “EL 200” debossed on one side | |
| Score | No score | No score | |
| Color | Light pink | Light orange | |
| Shape | Oblong | Oblong | |
| Dimension | 14 mm | 15 mm | |
| Mfg by |
AbbVie Inc. North Chicago, IL 60064 |
||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N210450 | 1 | 6872728 | January 25, 2021 | DS | DP | - | - | Download |
| N210450 | 1 | 7056927 | September 10, 2024 | DS | DP | - | - | Download |
| N210450 | 1 | 7176211 | July 6, 2024 | - | - | U-2360 | - | Download |
| N210450 | 1 | 7179815 | March 7, 2021 | - | - | U-2360 | - | Download |
| N210450 | 1 | 7419983 | July 6, 2024 | DS | DP | U-2360 | - | Download |
| N210450 | 1 | 7462625 | January 25, 2021 | DS | DP | U-2360 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| USP Apparatus 2 (paddle) | 50 | 0.05 M sodium phosphate (pH 6.8) | 900 mL | Q point at 30 min (For 150 mg) Q point at 45 min (For 200 mg) | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
