| Active Ingredient | DORAVIRINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIFELTRO | 210806 | MERCK SHARP DOHME | TABLET;ORAL | 100MG | 100MG | August 30, 2018 | August 30, 2023 | _ | Type 1 - New Molecular Entity | STANDARD | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
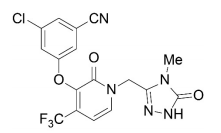 |
| Chemical Name | 3-chloro-5-[[1-[(4,5-dihydro-4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-1,2-dihydro-2-oxo-4-(trifluoromethyl)-3-pyridinyl]oxy]benzonitrile |
| CAS No | 1338225-97-0 |
| Molecular Formula | C17H11ClF3N5O3 |
| Molecular Weight | 425.75 |
| Appearance | White t off white crystalline powder |
| Solubility | - |
| Water Solubility | Practically insoluble in water |
| Polymorphism | Polymorphism has been observed for doravirine. Anhydrous Form II manufactured by the process described in this submission is crystalline as determined by XRPD methods |
| pKa (Strongest Acidic) | 8.26 |
| pKa (Strongest Basic) | 1.32 |
| Log P | 3.47 |
| Identification | IR, HPLC |
| Degradation | - |
| Hygroscopic | Non-hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | Doravirine is synthesized using commercially available well defined starting materials with acceptable specifications. The synthetic route has been demonstrated at multiple scales, from laboratory to commercial, within the ranges specified and has been shown to produce doravirine active substance meeting all in-process and release specifications. The selection of regulatory starting materials was discussed in connection with a CHMP Scientific Advice received in 2016. The definition of starting materials is considered justified and acceptable. The control of raw materials including the starting materials is sufficient. The in-process controls and the critical process parameter have been acceptably described and are considered adequate.The control of the isolated intermediates is satisfactory. |
| Parameters | Details |
|---|---|
| Indications and Usage | PIFELTRO, a non-nucleoside reverse transcriptase inhibitor (NNRTI),is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history |
| Dosage and Administration |
Recommended dosage: One tablet taken orally once daily with or without food in adult patients. Dosage adjustment with rifabutin: One tablet taken twice daily (approximately 12 hours apart) |
| Mechanism of action | Doravirine is a pyridinone non-nucleoside reverse transcriptase inhibitor of HIV-1 and inhibits HIV-1 replication by non-competitive inhibition of HIV-1 reverse transcriptase (RT). Doravirine does not inhibit the human cellular DNA polymerases α, ß, and mitochondrial DNA polymerase γ. |
| Absorption | - |
| Food Effect |
AUC Ratio: 1.16 (1.06, 1.26) Cmax Ratio: 1.03 (0.89, 1.19) C24 Ratio: 1.36 (1.19, 1.55) |
| Distribution |
Vdss (L): 60.5 Plasma Protein Binding: 76% |
| Metabolism | Primary Pathway(s): CYP3A |
| Elimination | - |
| Peak plasma time (Tmax) | 2 hours |
| Half life | 15 hours |
| Bioavailability | 64% |
| Age, gender | No clinically significant difference on the pharmacokinetics of doravirine were observed based on age (18 to 78 years of age), sex, and race/ethnicity, mild to severe renal impairment (creatinine clearance (CLcr) >15 mL/min, estimated by Cockcroft-Gault), or moderate hepatic impairment (Child-Pugh B). The pharmacokinetics of doravirine in patients with end-stage renal disease or undergoing dialysis, severe hepatic impairment (Child-Pugh C), or <18 years of age is unknown. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 100 MG |
| Excipients used | Colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate,lactose monohydrate (222 mg), magnesium stearate, and microcrystalline cellulose |
| Composition of coating material | Hypromellose, lactose monohydrate, titanium dioxide, and triacetin. The coated tablets are polished with carnauba wax |
| Composition of caspule shell | - |
| Pharmaceutical Development |
Key physical properties of the active substance were taken into account during the pharmaceutical development. A risk based approach was used to demonstrate the doravirine in final formulation would remain physically stable over the proposed shelf life. The dissolution method development has been adequately described and the choice of the dissolution medium, apparatus and agitation speed has been justified. The discriminating ability of the method has been sufficiently demonstrated. The dissolution method was shown to be discriminative. |
| Manufacture of the product | The process is considered to be a standard manufacturing process consisting of conventional manufacturing steps including spray dry blending, lubrication, roller compaction, tablet compression ,film coating and packaging. |
| Tablet / Capsule Image |
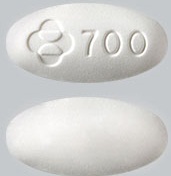
|
| Appearance | White, oval-shaped and film-coated, and is debossed with the corporate logo and 700 on one side and plain on the other side |
| Imprint code / Engraving / Debossment | Debossed with the corporate logo and 700 on one side |
| Score | No score |
| Color | White |
| Shape | Oval |
| Dimension | 19 mm |
| Mfg by | - |
| Mfg for | Merck Sharp & Dohme Corp |
| Marketed by | - |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N210806 | 1 | 8486975 | October 7, 2031 | DS | DP | U-2394 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| USP II (Paddle) | 75 RPM | 25 mM Phosphate buffer (pH 6.8) with 3% w/v Polysorbate 80 37 ± 0.5°C | 900 mL | Q point in 30 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | PIFELTRO | Download |
| UK | PIFELTRO | Download |
| US | PIFELTRO | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
