| Active Ingredient | DEXLANSOPRAZOLE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEXILANT SOLUTAB | NDA#208056 | TAKEDA PHARMS USA | TABLET, ORALLY DISINTEGRATING, DELAYED RELEASE;ORAL | 30MG | 30MG | January 26, 2016 | - | - | Type 3 - New Dosage Form | STANDARD | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
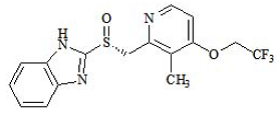 |
| Chemical Name | (+)-2-[(R)-{[3-methyl4-(2,2,2-trifluoroethoxy)pyridin-2-yl] methyl} sulfinyl]-1H-benzimidazole Dexlansoprazole is the R-enantiomer of lansoprazole (a racemic mixture of the R-and Senantiomers). |
| CAS No | - |
| Molecular Formula | C16H14F3N3O2S |
| Molecular Weight | 369.36 |
| Appearance | Dexlansoprazole is a white to nearly white crystalline powder. |
| Solubility | Dexlansoprazole is freely soluble in dimethylformamide, methanol, dichloromethane, ethanol, and ethyl acetate; and soluble in acetonitrile; slightly soluble in ether; and very slightly soluble in water; and practically insoluble in hexane. |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | - |
| pKa (Strongest Basic) | - |
| Log P | - |
| Identification | - |
| Degradation | Dexlansoprazole is more stable in neutral and alkaline conditions than acidic conditions. |
| Hygroscopic | - |
| Photostability study | Dexlansoprazole is stable when exposed to light. |
| Melting Point | melts with decomposition at 140°C |
| BCS Class | II |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | DEXILANT delayed-release capsules (DEXILANT capsules) are indicated in adults for healing of all grades of erosive esophagitis (EE) for up to eight weeks. DEXILANT capsules and DEXILANT SoluTab delayed-release orally disintegrating tablets (DEXILANT SoluTab) are indicated in adults to maintain healing of EE and relief of heartburn for up to six months. DEXILANT capsules and DEXILANT SoluTab are indicated in adults for the treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD) for four weeks. |
| Dosage and Administration | Refer FDA Label |
| Mechanism of action | Dexlansoprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that suppress gastric acid secretion by specific inhibition of the (H+, K+)-ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (-proton) pump within the parietal cell, dexlansoprazole has been characterized as a gastric proton-pump inhibitor, in that it blocks the final step of acid production. |
| Absorption |
After oral administration of DEXILANT 30 mg or 60 mg capsules to healthy subjects and symptomatic GERD patients, mean Cmax and AUC values of dexlansoprazole increased approximately dose proportionally. When granules of DEXILANT 60 mg capsules are mixed with water and dosed via NG tube or orally via syringe, the bioavailability (Cmax and AUC) of dexlansoprazole was similar to that when DEXILANT 60 mg was administered as an intact capsule. After oral administration of DEXILANT SoluTab 30 mg tablet to healthy adults under fasting condition, median time (Tmax) to peak plasma concentrations (Cmax) of dexlansoprazole was 4 hours and ranged from 1 to 6 hours, the Cmax was 688 ng/mL (CV of 49%) and AUC was 2866 ng·h/mL (CV of 77%). The bioavailability (Cmax and AUC) of dexlansoprazole was similar when DEXILANT SoluTab 30 mg tablets were mixed with water and administered via oral syringe, NG tube, or swallowed intact with water compared to DEXILANT SoluTab 30 mg tablets administered on the tongue, allowed to disintegrate and swallowed without water under fasting conditions in healthy subjects. Two 30 mg DEXILANT SoluTab are not interchangeable with one 60 mg DEXILANT capsule because systemic exposure is lower. |
| Food Effect |
In food-effect studies in healthy subjects receiving DEXILANT capsules under various fed conditions compared to fasting, increases in Cmax ranged from 12% to 55%, increases in AUC ranged from 9% to 37%, and Tmax varied (ranging from a decrease of 0.7 hours to an increase of three hours) In healthy adults, a concomitant administration of a standard high-fat breakfast contained approximately 800 to 1000 total calories, with 50% of calories being derived from fat content delayed the absorption of dexlansoprazole from DEXILANT SoluTab 30 mg tablet resulting in a median Tmax of 6 hours and decreased the Cmax on average by 38%. Dexlansoprazole AUC was not affected by food |
| Distribution | Plasma protein binding of dexlansoprazole ranged from 96% to 99% in healthy subjects and was independent of concentration from 0.01 to 20 mcg/mL. The apparent volume of distribution (Vz/F) after multiple doses in symptomatic GERD patients was 40 L. |
| Metabolism |
Dexlansoprazole is extensively metabolized in the liver by oxidation, reduction, and subsequent formation of sulfate, glucuronide and glutathione conjugates to inactive metabolites. Oxidative metabolites are formed by the cytochrome P450 (CYP) enzyme system including hydroxylation mainly by CYP2C19, and oxidation to the sulfone by CYP3A4. CYP2C19 is a polymorphic liver enzyme which exhibits three phenotypes in the metabolism of CYP2C19 substrates: extensive metabolizers (*1/*1), intermediate metabolizers (*1/mutant) and poor metabolizers (mutant/mutant). Dexlansoprazole is the major circulating component in plasma regardless of CYP2C19 metabolizer status. In CYP2C19 intermediate and extensive metabolizers, the major plasma metabolites are 5hydroxy dexlansoprazole and its glucuronide conjugate, while in CYP2C19 poor metabolizers dexlansoprazole sulfone is the major plasma metabolite. |
| Elimination | Following the administration of DEXILANT capsule, no unchanged dexlansoprazole is excreted in urine. Following the administration of [14C] dexlansoprazole to six healthy male subjects, approximately 50.7% (standard deviation (SD): 9.0%) of the administered radioactivity was excreted in urine and 47.6% (SD: 7.3%) in the feces. Apparent clearance (CL/F) in healthy subjects was 11.4 to 11.6 L/hour, respectively, after five days of 30 or 60 mg once daily administration. |
| Peak plasma time (Tmax) | 4 hours |
| Half life | - |
| Bioavailability | - |
| Age, gender |
The terminal elimination half-life of dexlansoprazole is significantly increased in geriatric subjects compared to younger subjects (2.2 and 1.5 hours, respectively). Dexlansoprazole exhibited higher systemic exposure (AUC) in geriatric subjects (34% higher) than younger subjects. In a study of 12 male and 12 female healthy subjects who received a single oral dose of DEXILANT 60 mg capsules, females had higher systemic exposure (AUC) (43% higher) than males. This difference in exposure between males and female does not represent a significant safety concern. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 22607 | A | II | March 12, 2009 | DR REDDYS LABORATORIES LTD (Amorphous) |
| 23380 | A | II | December 16, 2009 | AMINO CHEMICALS LTD |
| 23655 | A | II | March 22, 2010 | DR REDDYS LABORATORIES LTD (Crystalline) |
| 23722 | A | II | April 19, 2010 | MSN LABORATORIES PRIVATE LTD |
| 24503 | A | II | December 21, 2010 | MYLAN LABORATORIES LTD |
| 24875 | A | II | April 15, 2011 | AMINO CHEMICALS LTD |
| 25235 | A | II | August 31, 2011 | ORCHID CHEMICALS AND PHARMACEUTICALS LTD |
| 26087 | A | II | May 23, 2012 | RAKS PHARMA PVT LTD |
| 27834 | A | II | February 6, 2014 | LUPIN LTD |
| 28052 | A | II | March 28, 2014 | JUBILANT GENERICS LTD |
| 29035 | A | II | March 30, 2015 | APOTEX PHARMACHEM INC |
| 29346 | A | II | May 19, 2015 | WATERSTONE PHARMACEUTICALS HUBEI CO LTD |
| Parameters | Details | |||
|---|---|---|---|---|
| Strength | 30MG Capsule | 60MG Capsule | 30MG Tablet | |
| Excipients used | Delayed-release capsules: Each capsule contains enteric-coated granules consisting of dexlansoprazole (active ingredient) and the following inactive ingredients: sugar spheres, magnesium carbonate, sucrose, low-substituted hydroxypropyl cellulose, titanium dioxide, hydroxypropyl cellulose, hypromellose 2910, talc, Methacrylic acid-ethyl acrylate copolymer (1:1) dispersion 30 per cent (Methacrylic acid units, Ethyl acrylate units, Methacrylic acid-methyl methacrylate copolymer (1:1), Methacrylic acid-methyl methacrylate copolymer (1:2), polyethylene glycol 8000, triethyl citrate, polysorbate 80, and colloidal silicon dioxide. |
Delayed-release orally disintegrating tablets: lactose monohydrate-microcrystalline cellulose spheres, magnesium carbonate, low-substituted hydroxypropyl cellulose, hydroxypropyl cellulose, hypromellose, talc, titanium dioxide, mannitol, methacrylic acid copolymer, ethyl acrylate methyl methacrylate copolymer, polysorbate 80, glyceryl monostearate, triethyl citrate, anhydrous citric acid, ferric oxide, red; ferric oxide, yellow; polyethylene glycol 8000, methylacrylate methylmethacrylate methacrylic acid copolymer, microcrystalline cellulose, crospovidone, sucralose, strawberry durarome, and magnesium stearate. | ||
| Composition of coating material | - | |||
| Composition of caspule shell |
hypromellose, carrageenan and potassium chloride. Based on the capsule shell color, blue contains FD&C Blue No. 2 aluminum lake; gray contains black ferric oxide; and both contain titanium dioxide. Printing ink: Iron oxide, red (E172), Iron oxide, yellow (E172), Indigotine (E132), Carnauba wax, Shellac, Glycerol mono-oleate |
- | ||
| Pharmaceutical Development | Dexlansoprazole is supplied for oral administration as a dual delayed-release formulation in capsules and orally disintegrating tablets. The capsules and tablets contain dexlansoprazole in a mixture of two types of enteric-coated granules with different pH-dependent dissolution profiles. | |||
| Manufacture of the product | - | |||
| Tablet / Capsule Image |
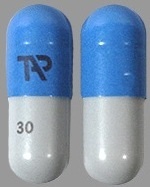
|
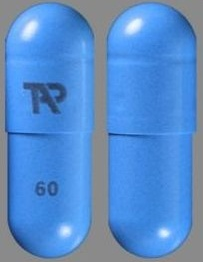
|

|
|
| Appearance | opaque, blue and gray capsule imprinted with TAP and “30” | opaque, blue capsule imprinted with TAP and “60” | white to yellowish-white, round, tablet containing orange to dark brown speckles, with “D30” debossed on one side | |
| Imprint code / Engraving / Debossment | TAP and “30” | TAP and “60” | “D30” debossed on one side and Plain on other side | |
| Score | no score | no score | no score | |
| Color | BLUE (opaque) , GRAY (opaque) | BLUE (o paque) | WHITE (white to yello wish white unco ated tablets with o range to dark bro wn speckles) STRAWBERRY Flavour | |
| Shape | Capsule | Capsule | ROUND | |
| Dimension | 16mm | 18mm | 13mm | |
| Mfg by | Takeda Pharmaceuticals America, Inc.(US) | |||
| Mfg for | - | |||
| Marketed by | Takeda Pharmaceuticals America, Inc.(US) | |||
| Distributed by | Takeda Pharmaceuticals America, Inc.(US) | |||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N208056 | 1 | 6328994 | May 17, 2019 | - | DP | - | - | Download |
| N208057 | 1 | 6328994*PED | November 17, 2019 | - | - | - | - | |
| N208058 | 1 | 6462058 | June 15, 2020 | DS | DP | U-950 U-951 | - | Download |
| N208059 | 1 | 6462058*PED | December 15, 2020 | - | - | - | - | |
| N208060 | 1 | 6664276 | January 30, 2023 | DS | DP | U-950 U-951 | - | Download |
| N208061 | 1 | 6664276*PED | July 30, 2023 | - | - | - | - | |
| N208062 | 1 | 6939971 | June 15, 2020 | - | - | U-950 U-951 | - | Download |
| N208063 | 1 | 6939971*PED | December 15, 2020 | - | - | - | - | |
| N208064 | 1 | 7285668 | June 15, 2020 | DS | - | - | - | Download |
| N208065 | 1 | 7285668*PED | December 15, 2020 | - | - | - | - | |
| N208066 | 1 | 7399485 | May 26, 2018 | - | DP | - | - | Download |
| N208067 | 1 | 7399485*PED | November 26, 2018 | - | - | - | - | |
| N208068 | 1 | 7431942 | May 17, 2019 | - | DP | - | - | Download |
| N208069 | 1 | 7431942*PED | November 17, 2019 | - | - | - | - | |
| N208070 | 1 | 7875292 | May 17, 2019 | - | DP | - | - | Download |
| N208071 | 1 | 7875292*PED | November 17, 2019 | - | - | - | - | |
| N208072 | 1 | 8461187 | January 17, 2026 | - | DP | - | - | Download |
| N208073 | 1 | 8461187*PED | July 17, 2026 | - | - | - | - | |
| N208074 | 1 | 8784885 | October 15, 2023 | - | DP | - | - | Download |
| N208075 | 1 | 8784885*PED | April 15, 2024 | - | - | - | - | |
| N208076 | 1 | 8871273 | January 11, 2028 | - | DP | - | - | Download |
| N208077 | 1 | 8871273*PED | July 11, 2028 | - | - | - | - | |
| N208078 | 1 | 9011926 | February 24, 2026 | - | DP | - | - | Download |
| N208079 | 1 | 9145389 | June 15, 2020 | DS | DP | - | - | |
| N208080 | 1 | 9238029 | January 17, 2026 | - | DP | - | - | Download |
| N208081 | 1 | 9241910 | March 10, 2029 | - | DP | - | - |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| Capsule (Delayed Release) I (Basket) | 100 | Acid Stage: 0.1 N HCl, Buffer Stage: pH 7.0 Phosphate Buffer with 5 mM SLS | Acid Stage: 500; Buffer stage: 900 | Acid Stage: 120; Buffer Stage: 10, 20, 40, 50, 60 , 75, 105 and 120 | August 5, 2010 |
| Tablet (Delayed Release, Orally Disintegrating) I (Basket -100 mesh) | 100 | Acid Stage: 0.1 N HCl; Buffer Stage: pH 7.2 Phosphate Buffer with 5 mM Sodium lauryl sulfate | Acid Stage: 500 mL; Buffer Stage: 900 mL | Acid Stage: 120; Buffer Stage: 10, 15, 20, 30, 50, 60, 75 and 90 | July 28, 2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | |
| FDA Pharmacology Review(s) | |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | |
| FDA BE Recommendation | Download |
| European Public Assessment Report |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | - | |
| UK | - | |
| US | DEXILANT SOLUTAB | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov, www.drug.com |
