| Active Ingredient | DELAFLOXACIN |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAXDELA | NDA 208610 | MELINTA THERAPEUTICS INC | TABLET;ORAL | 450MG | TBD | June 19, 2017 | June 19, 2022 | _ | Type 1 - New Molecular Entity | PRIORITY | 450MG | None |
| Parameters | Details |
|---|---|
| Structural Formula |
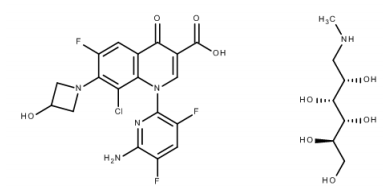 |
| Chemical Name | 1-Deoxy-1(methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)4-oxo-1,4-dihydroquinoline-3-carboxylate (salt), |
| CAS No | 189279-58-1 |
| Molecular Formula | C18H12CLF3N4O4 C7H17NO5 |
| Molecular Weight | Meglumine salt has a molecular weight of 635.97 g/mol, whereas the molecular weight of the delafloxacin free acid is 440.76 g/mol. |
| Appearance | - |
| Solubility | - |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | 5.62 |
| pKa (Strongest Basic) | -1.3 |
| Log P | 1.67 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | BAXDELA is a fluoroquinolone antibacterial indicated in adults for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible bacteria. To reduce the development of drug-resistant bacteria and maintain the effectiveness of BAXDELA and other antibacterial drugs, BAXDELA should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. |
| Dosage and Administration |
Administer BAXDELA for injection 300 mg by intravenous infusion over 60 minutes, every 12 hours, or a 450-mg BAXDELA tablet orally every 12 hours for 5 to 14 days total duration. Dosage for patients with renal impairment is based on the estimated glomerular filtration rate (eGFR) Refer FDA PIL for more details |
| Mechanism of action | Delafloxacin belongs to the fluoroquinolone class of antibacterial drugs and is anionic in nature. The antibacterial activity of delafloxacin is due to the inhibition of both bacterial topoisomerase IV and DNA gyrase (topoisomerase II) enzymes which are required for bacterial DNA replication, transcription, repair, and recombination. Delafloxacin exhibits a concentration-dependent bactericidal activity against gram-positive and gram-negative bacteria in vitro. |
| Absorption | The absolute bioavailability for BAXDELA 450 mg oral tablet administered as a single dose was 58.8%. The AUC of delafloxacin following administration of a single 450 mg oral (tablet) dose was comparable to that following a single 300 mg intravenous dose. The Cmax of delafloxacin was achieved within about 1 hour after oral administration under fasting condition. |
| Food Effect | Food (kcal:917, Fat: 58.5%, Protein: 15.4%, Carbohydrate: 26.2%).did not affect the bioavailability of delafloxacin |
| Distribution | The steady state volume of distribution of delafloxacin is 30–48 L which approximates total body water. The plasma protein binding of delafloxacin is approximately 84%; delafloxacin primarily binds to albumin. Plasma protein binding of delafloxacin is not significantly affected by renal impairment. |
| Metabolism | Glucuronidation of delafloxacin is the primary metabolic pathway with oxidative metabolism representing about 1% of an administered dose. The glucuronidation of delafloxacin is mediated mainly by UGT1A1, UGT1A3, and UGT2B15. Unchanged parent drug is the predominant component in plasma. There are no significant circulating metabolites in humans. |
| Elimination |
In a mass balance study, the mean half-life for delafloxacin was 3.7 hours (SD 0.7 hour) after a single dose intravenous administration. The mean half-life values for delafloxacin ranged from 4.2 to 8.5 hours following multiple oral administrations. Following administration of a single 300 mg intravenous dose of BAXDELA, the mean clearance (CL) of delafloxacin was 16.3 L/h (SD 3.7 L/h), and the renal clearance (CLr) of delafloxacin accounts for 35-45% of the total clearance. After single intravenous dose of 14C-labeled delafloxacin, 65% of the radioactivity was excreted in urine as unchanged delafloxacin and glucuronide metabolites and 28% was excreted in feces as unchanged delafloxacin. Following a single oral dose of 14C-labeled delafloxacin, 50% of the radioactivity was excreted in urine as unchanged delafloxacin and glucuronide metabolites and 48% was excreted in feces as unchanged delafloxacin. |
| Peak plasma time (Tmax) | Single dose 450 mg: 0.75 (0.5, 4.0) Steady State 450 mg Q12h :- 1.00 (0.50, 6.00) |
| Half life | The mean half-life for delafloxacin was 3.7 hours (SD 0.7 hour) after a single dose intravenous administration. The mean half-life values for delafloxacin ranged from 4.2 to 8.5 hours following multiple oral administrations. |
| Bioavailability | 59% |
| Age, gender | Based on a population pharmacokinetic analysis, the pharmacokinetics of delafloxacin were not significantly impacted by age, sex, race, weight, body mass index, and disease state (ABSSSI). |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 450 MG |
| Excipients used | Citric acid anhydrous (5.5 mg); crospovidone (109 mg);magnesium stearate (10 mg); microcrystalline cellulose (417 mg); povidone (34 mg); sodium bicarbonate (140 mg); sodium phosphate monobasic monohydrate (5.5 mg). |
| Composition of coating material | - |
| Composition of caspule shell | - |
| Pharmaceutical Development |
Each BAXDELA tablet for oral use contains 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine) |
| Manufacture of the product | - |
| Tablet / Capsule Image | |
| Appearance | Each modified capsule-shaped tablet in beige to mottled beige color is debossed with RX3341 on one side |
| Imprint code / Engraving / Debossment | Debossed with RX3341 on one side |
| Score | No score |
| Color | Beige to mottled beige color |
| Shape | Capsule-shaped |
| Dimension | 21 mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | - |
| Distributed by | Melinta Therapeutics, Inc. |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N208611 | 1 | 7728143 | November 20, 2027 | DS | - | - | - | Download |
| N208611 | 1 | 8252813 | October 2, 2026 | - | DP | U-2028 | - | Download |
| N208611 | 1 | 8273892 | August 6, 2026 | DS | - | - | - | Download |
| N208611 | 1 | 8497378 | December 28, 2029 | DS | - | - | - | Download |
| N208611 | 1 | 8648093 | October 7, 2025 | - | DP | U-2028 | - | Download |
| N208611 | 1 | 8871938 | September 23, 2029 | DS | - | - | - | Download |
| N208611 | 1 | 9539250 | October 7, 2025 | DS | DP | U-2028 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II | 75 | pH 4.5 Sodium acetate buffer (SBOA media) | 500 | Acceptace criteria revised to Q at 15 min form 10 min | (SBOA media and condition) |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
