| Active Ingredient | DEFLAZACORT |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMFLAZA | 208684 | MARATHON PHARMACEUTICALS LLC | TABLET;ORAL | 6 MG, 18 MG, 30 MG, 36 MG | TBD | February 9, 2017 | Feb 9, 2022 | Feb 9, 2024 | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
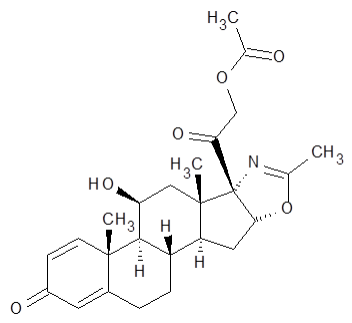 |
| Chemical Name | (11β,16β)-21-(acetyloxy)11-hydroxy-2'-methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione |
| CAS No | 14484-47-0 |
| Molecular Formula | C25H31NO6 |
| Molecular Weight | 441.517 |
| Appearance | White to off white, odorless fine powder |
| Solubility | Freely soluble in acetic acid and dichloromethane and soluble in methanol and acetone |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | 14.74 (Predicted) |
| pKa (Strongest Basic) | 0.48 (Predicted) |
| Log P | 2.56 (Predicted) |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | I |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | EMFLAZA is a corticosteroid indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older |
| Dosage and Administration |
The recommended once-daily dosage is approximately 0.9 mg/kg/day administered orally Discontinue gradually when administered for more than a few days |
| Mechanism of action | Deflazacort is a corticosteroid prodrug, whose active metabolite, 21-desDFZ, acts through the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. The precise mechanism by which deflazacort exerts its therapeutic effects in patients with DMD is unknown. |
| Absorption | After oral administration in the fasted state, the median Tmax with deflazacort tablets or suspension is about 1 hour (range 0.25 to 2 hours). |
| Food Effect | Co-administration of deflazacort tablets with a high-fat meal reduced Cmax by about 30% and delayed Tmax by one hour, relative to administration under fasting conditions, but there was no effect on the overall systemic absorption as measured by AUC. The bioavailability of deflazacort tablets was similar to that of the oral suspension. The administration of deflazacort with food or crushed in applesauce did not affect the absorption and bioavailability of deflazacort. |
| Distribution | The protein binding of the active metabolite of deflazacort is about 40%. |
| Metabolism | Deflazacort is rapidly converted to the active metabolite 21-desDFZ by esterases after oral administration. 21-desDFZ is further metabolized by CYP3A4 to several other inactive metabolites. |
| Elimination | Urinary excretion is the predominant route of deflazacort elimination (about 68% of the dose), and the elimination is almost completed by 24 hours post dose. 21-desDFZ accounts for 18% of the eliminated drug in the urine. |
| Peak plasma time (Tmax) | 1 hour (range 0.25 to 2 hours) |
| Half life | - |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 24691 | A | II | March 2, 2011 | SYMBIOTEC PHARMALAB PRIVATE LTD |
| 30313 | A | II | March 2, 2016 | STERLING SPA |
| 4922 | I | II | March 17, 1983 | SCHERING AG |
| Parameters | Details | ||||
|---|---|---|---|---|---|
| Strength | 6 MG | 36 MG | 18 MG | 30 MG | |
| Excipients used | Colloidal silicon dioxide, lactose monohydrate, magnesium stearate, and pre-gelatinized corn starch | ||||
| Composition of coating material | - | ||||
| Composition of caspule shell | - | ||||
| Pharmaceutical Development | - | ||||
| Manufacture of the product | - | ||||
| Tablet / Capsule Image |

|

|

|

|
|
| Appearance | White, round with “6” debossed on one side | White, oval with “36” debossed on one side | White, round with “18” debossed on one side | White, oval with “30” debossed on one side | |
| Imprint code / Engraving / Debossment | “6” debossed on one side | “36” debossed on one side | “18” debossed on one side | “30” debossed on one side | |
| Score | No score | No score | No score | No score | |
| Color | White | White | White | White | |
| Shape | Round | Oval | Round | Oval | |
| Dimension | 6 mm | 17 mm | 10 mm | 15 mm | |
| Mfg by | - | ||||
| Mfg for | Marathon Pharmaceuticals, LLC | ||||
| Marketed by | - | ||||
| Distributed by | - | ||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| Not Available | |||||
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
