| Active Ingredient | CRIZOTINIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XALKORI | (NDA) 202570 | PF PRISM CV | CAPSULE;ORAL | 200MG, 250MG | 200MG, 250MG (RS) | August 26, 2011 | - | August 26, 2018 March 11, 2023 | 1 New molecular entity (NME) | P Priority review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
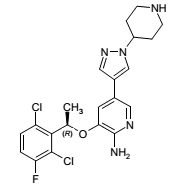 |
| Chemical Name | (R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine |
| CAS No | 877399-52-5 |
| Molecular Formula | C21H22Cl2FN5O |
| Molecular Weight | 450.34 daltons |
| Appearance | a white to pale-yellow powder |
| Solubility | The solubility of crizotinib in aqueous media decreases over the range pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65. |
| Water Solubility | 0.00611 mg/mL (Predicted) |
| Polymorphism | Only one crystalline form has been found, this is the thermodynamically stable form A, no other crystalline form has been found. |
| pKa (Strongest Acidic) | 9.4 (piperidinium cation)and 5.6 (pyridiniumcation) |
| pKa (Strongest Basic) | 10.12 (Predicted) |
| Log P | 1.83 |
| Identification | FTIR |
| Degradation | Stress studies were performed at high temperatures and no significant degradation was observed after 14 days stored at 100°C. Minor degradation was observed when crizotinib was exposed to strongly acidic, strongly basic, intense light and oxidative conditions. |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | IV |
| Manufacture of API | The synthetic process of the active substance Crizotinib is using well characterised and commercially available starting materials. The batch size is based on input of starting materials. The description of the manufacturing process is sufficiently detailed. The applicant has used risk assessments and design of experiments to identify the critical process parameters and adequate in process controls have been put in place. Proven acceptable ranges have been established and normal operating ranges for critical parameters have been provided. The justification of the starting materials and their specification is based on their manufacturing process, the impurities that can be generated and their fate in the crizotinib synthesis. |
| Parameters | Details |
|---|---|
| Indications and Usage | XALKORI is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK)-positive as detected by an FDA-approved test. XALKORI is indicated for the treatment of patients with metastatic NSCLC whose tumors are ROS1-positive. |
| Dosage and Administration |
The recommended dose of XALKORI is 250 mg orally, twice daily until disease progression or no longer tolerated by the patient. The recommended dose of XALKORI in patients with severe renal impairment [creatinine clearance (CLcr) <30 mL/min] not requiring dialysis is 250 mg orally, once daily. XALKORI may be taken with or without food. Swallow capsules whole. If a dose of XALKORI is missed, make up that dose unless the next dose is due within 6 hours. If vomiting occurs after taking a dose of XALKORI, take the next dose at the regular time. |
| Mechanism of action | Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, HepatocyteGrowth Factor Receptor (HGFR, c-Met), ROS1 (c-ros), and Recepteur d’Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the gene’s expression and signaling which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrated concentration-dependent inhibition of ALK, ROS1, and c-Met phosphorylation in cell-based assays using tumor cell lines and demonstrated antitumor activity in mice bearing tumor xenografts that expressed echinoderm microtubule-associated protein-like 4 (EML4)- or nucleophosmin (NPM)-ALK fusion proteins or c-Met. |
| Absorption | Following a single oral dose, crizotinib was absorbed with median time to achieve peak concentration of 4 to 6 hours. Following crizotinib 250 mg twice daily, steady state was reached within 15 days and remained stable, with a median accumulation ratio of 4.8. Steady-state systemic exposure [observed minimum concentration (Cmin) and AUC] appeared to increase in a greater than dose-proportional manner over the dose range of 200-300 mg twice daily. |
| Food Effect | A high-fat meal reduced crizotinib AUC from time zero to infinity (AUCinf) and maximum observed plasma concentration (Cmax) by approximately 14%. XALKORI can be administered with or without food. |
| Distribution |
The geometric mean volume of distribution (Vss) of crizotinib was 1772 L following intravenous administration of a 50 mg dose, indicating extensive distribution into tissues from the plasma. Binding of crizotinib to human plasma proteins in vitro is 91% and is independent of drug concentration. In vitro studies suggested that crizotinib is a substrate for P-glycoprotein (P-gp). The blood-to-plasma concentration ratio is approximately 1. |
| Metabolism | Crizotinib is predominantly metabolized by CYP3A4/5. The primary metabolic pathways in humans were oxidation of the piperidine ring to crizotinib lactam and O-dealkylation, with subsequent Phase 2 conjugation of O-dealkylated metabolites. |
| Elimination |
Following single doses of crizotinib, the mean apparent plasma terminal half-life ofcrizotinib was 42 hours in patients. Following the administration of a single 250 mg radiolabeled crizotinib dose to healthy subjects, 63% and 22% of the administered dose was recovered in feces and urine, respectively. Unchanged crizotinib represented approximately 53% and 2.3% of the administered dose in feces and urine, respectively. The mean apparent clearance (CL/F) of crizotinib was lower at steady state (60 L/h) after 250 mg twice daily than after a single 250 mg oral dose (100 L/h), which was likely due to autoinhibition of CYP3A by crizotinib after multiple dosing. |
| Peak plasma time (Tmax) | 4 to 6 hours |
| Half life | 42 hours |
| Bioavailability | The mean absolute bioavailability of crizotinib was 43% (range: 32% to 66%) following a single 250 mg oral dose. |
| Age, gender |
No clinically relevant difference in the exposure of crizotinib between Asian patients (n=523) and non-Asian patients (n=691). Age has no effect on the exposure of crizotinib based on the population pharmacokinetic analysis. No clinically relevant effect of body weight or gender on the exposure of crizotinib based on the population pharmacokinetic analysis. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||
|---|---|---|---|
| Strength | 200MG | 250MG | |
| Excipients used | colloidal silicon dioxide (2mg), microcrystalline cellulose (83mg), anhydrous dibasic calcium phosphate (83mg), sodium starch glycolate (20mg), magnesium stearate (12mg) | colloidal silicon dioxide (2.5mg), microcrystalline cellulose (103.75mg), anhydrous dibasic calcium phosphate (103.75mg), sodium starch glycolate (25mg), magnesium stearate (15mg) | |
| Composition of coating material | - | ||
| Composition of caspule shell |
The pink opaque hard gelatin capsule shell components contain gelatin, titanium dioxide, and red iron oxide. The white opaque hard gelatin capsule shell components contain gelatin and titanium dioxide. The printing ink contains shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide. |
||
| Pharmaceutical Development |
The aim of the development was to obtain an immediate release hard gelatin capsule to deliver 200 mg and 250 mg crizotinib as a single unit dose. The initial dosage form used during early Phase 1 clinical studies was a powder in capsule (PIC), consisting of crizotinib in a hard gelatin capsule shell. Then a tablet dosage form was developed containing 50 mg and 100 mg crizotinib (drug substance loading of 12.5%), to meet increased clinical demand for Phase 3 clinical studies. In vitro dissolution profiles for both the clinical tablet and PIC dosage forms demonstrated rapid release within 30 minutes in 0.1N HCl. In vivo performance was subsequently shown to be bioequivalent between the clinical tablet and PIC. However for manufacturability reasons, the crizotinib capsule form was developed and it was designed to be qualitatively similar to the clinical tablet formulation. The pharmaceutical development was based on the Quality by Design concept. |
||
| Manufacture of the product |
The manufacturing process is considered as standard, and adequate information has been provided. The main critical steps have been studied in detail andthe critical process parameters (CPPs) have been described adequately. . The target normal operating ranges and proven for these CPPs are considered acceptable. A satisfactory validation protocol was filed. |
||
| Tablet / Capsule Image |

|

|
|
| Appearance | hard gelatin capsule, size 1, white opaque body and pink opaque cap, printed with black ink “Pfizer” on the cap and “CRZ 200” on the body | hard gelatin capsule, size 0, pink opaque cap and body, printed with black ink “Pfizer” on the cap and “CRZ 250” on the body | |
| Imprint code / Engraving / Debossment | printed with black ink “Pfizer” on the cap and “CRZ 200” on the body | printed with black ink “Pfizer” on the cap and “CRZ 250” on the body | |
| Score | no score | no score | |
| Color | white opaque body and pink opaque cap | pink opaque cap and body | |
| Shape | Capsule | Capsule | |
| Dimension | Size 1 | Size 0 | |
| Mfg by | Pfizer labs (EU) | ||
| Mfg for | - | ||
| Marketed by | Pfizer labs (EU) | ||
| Distributed by | Pfizer labs (US) | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N202570 | 1 | 7230098 | August 26, 2025 | Y | - | - | - | Download |
| N202570 | 1 | 7825137 | May 12, 2027 | - | - | U - 1179 | - | Download |
| N202570 | 1 | 7858643 | October 8, 2029 | Y | Y | - | - | Download |
| N202570 | 1 | 8217057 | November 6, 2029 | Y | Y | - | - | Download |
| N202570 | 1 | 8785632 | March 1, 2025 | Y | - | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| I (Basket) | 100 | 0.1N HCl (degassed) | 900 | 5, 10, 15, 30 and 45 | April 14, 2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | XALKORI | Download |
| UK | XALKORI | Download |
| US | XALKORI | Download |
| Exclusivity Code: Exclusivity Expiration is M - 163: Sep 14, 2018. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
