| Active Ingredient | CLOBAZAM |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ONFI | (NDA) 202067 | LUNDBECK LLC | TABLET;ORAL | 5MG, 10MG, 20MG | 10MG, 20MG (RS) | October 21, 2011 | - | October 21, 2018 | 1 New molecular entity (NME) | S Standard review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
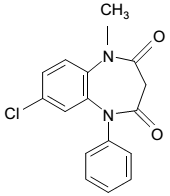 |
| Chemical Name | 7-Chloro-1-methyl-5-phenyl-1H-1,5 benzodiazepine-2,4(3H,5H)-dione |
| CAS No | 22316-47-8 |
| Molecular Formula | C16H13O2N2Cl |
| Molecular Weight | 300.7 |
| Appearance | a white or almost white, crystalline powder with a slightly bitter taste |
| Solubility | It is slightly soluble in water, sparingly soluble in ethanol, and freely soluble in methylene chloride. |
| Water Solubility | 188 mg/L |
| Polymorphism | - |
| pKa (Strongest Acidic) | 4.07 (Predicted) |
| pKa (Strongest Basic) | (Predicted) -6.7 |
| Log P | 2.12 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 182ºC to 185ºC |
| BCS Class | II |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | ONFI (clobazam) is indicated for the adjunctive treatment of seizures associated with Lennox-Gastautsyndrome (LGS) in patients 2 years of age or older. |
| Dosage and Administration | A daily dose of ONFI greater than 5 mg should be administered in divided doses twice daily; a 5 mg daily dose can be administered as a single dose. Dose patients according to body weight. Individualize dosing within each body weight group, based on clinical efficacy and tolerability. Do not proceed with dose escalation more rapidly than weekly, because serum concentrations of clobazam and its active metabolite require 5 and 9 days, respectively, to reach steady-state. |
| Mechanism of action | The exact mechanism of action for clobazam, a 1,5-benzodiazepine, is not fully understood but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor. |
| Absorption |
The peak plasma levels (Cmax) and the area under the curve (AUC) of clobazam are dose-proportional over the dose range of 10-80 mg following single- or multiple-dose administration of ONFI. Based on a population pharmacokinetic analysis, the pharmacokinetics of clobazam are linear from 5-160 mg/day. Clobazam is converted to N-desmethylclobazam which has about 1/5 the activity of clobazam. Clobazam is rapidly and extensively absorbed following oral administration. The time to peak concentrations (Tmax) of clobazam tablets under fasted conditions ranged from 0.5 to 4 hours after single- or multiple-dose administrations. The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. After single dose administration of the oral suspension under fasted conditions, the Tmax ranged from 0.5 to 2 hours. Based on exposure (Cmax and AUC) of clobazam, ONFI tablets and suspension were shown to have similar bioavailability under fasted conditions. |
| Food Effect | The administration of ONFI tablets with food or when crushed in applesauce doesnot affect absorption. Although not studied, the oral bioavailability of the oral suspension is unlikely to be affected under fed conditions. |
| Distribution | Clobazam is lipophilic and distributes rapidly throughout the body. The apparent volume of distribution at steady state was approximately 100 L. The in vitro plasma protein binding of clobazam and N-desmethylclobazam is approximately 80-90% and 70%, respectively. |
| Metabolism | Clobazam is extensively metabolized in the liver, with approximately 2% of the dose recovered in urine and 1% in feces as unchanged drug. The major metabolic pathway of clobazam involves N-demethylation, primarily by CYP3A4 and to a lesser extent by CYP2C19 and CYP2B6. N-desmethylclobazam, an active metabolite, is the major circulating metabolite in humans, and at therapeutic doses, plasma concentrations are 3-5 times higher than those of the parent compound. Based on animal and in vitro receptor binding data, estimates of the relative potency of N-desmethylclobazam compared to parent compound range from 1/5 to equal potency. N-desmethylclobazam is extensively metabolized, mainly by CYP2C19. N-desmethylclobazam and its metabolites comprise ~94% of the total drug-related components in urine. Following a single oral dose of radiolabeled drug, approximately 11% of the dose was excreted in the feces and approximately 82% was excreted in the urine. |
| Elimination | The polymorphic CYP2C19 is the major contributor to the metabolism of the pharmacologically active N-desmethylclobazam. In CYP2C19 poor metabolizers, levels of N-desmethylclobazam were 5 fold higher in plasma and 2- to 3-fold higher in the urine than in CYP2C19 extensive metabolizers. |
| Peak plasma time (Tmax) | 0.5 to 4 hours (Fasted), |
| Half life | The estimated mean elimination half-lives (t½) of clobazam and Ndesmethylclobazam were 36-42 hours and 71-82 hours, respectively. |
| Bioavailability | The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. |
| Age, gender |
Population pharmacokinetic analyses showed that the clearance of clobazam is lower in elderly subjects compared to other age groups (ages 2 to 64). Dosing should be adjusted in the elderly. Population pharmacokinetic analyses showedno difference in the clearance of clobazam between women and men. Population pharmacokinetic analyses including Caucasian (75%), African American (15%), and Asian (9%) subjects showed that there is no evidence of clinically significant effect ofrace on the clearance of clobazam. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 18080 | I | II | February 10, 2005 | FIS FABBRICA ITALIANA SINTETICI SPA |
| 18340 | A | II | May 9, 2005 | SANOFI AVENTIS DEUTSCHLAND GMBH |
| 24728 | A | II | February 28, 2011 | FIS FABBRICA ITALIANA SINTETICI SPA |
| 29061 | A | II | January 27, 2015 | CAMBREX PROFARMACO MILANO SRL |
| 29164 | A | II | March 28, 2015 | PIRAMAL ENTERPRISES LTD |
| 29351 | A | II | February 6, 2015 | HONOUR LAB LTD |
| 29407 | A | II | May 29, 2015 | CENTAUR PHARMACEUTICALS PRIVATE LTD |
| 30541 | A | II | June 2, 2016 | RAKS PHARMA PVT LTD |
| 30875 | A | II | September 2, 2016 | MSN LIFE SCIENCES PRIVATE LTD |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 10MG | 20MG | |
| Excipients used | corn starch, lactose monohydrate, magnesium stearate, silicon dioxide, and talc | ||
| Composition of coating material | - | ||
| Composition of caspule shell | - | ||
| Pharmaceutical Development | - | ||
| Manufacture of the product | - | ||
| Tablet / Capsule Image |
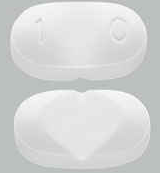
|
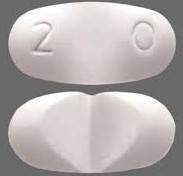
|
|
| Appearance | a white to off-white, oval tablet with a functional score on one side and either a “1” and “0” debossed on the other side | a white to off-white, oval tablet with a functional score on one side and either a “2” and “0” debossed on the other side | |
| Imprint code / Engraving / Debossment | score on one side and either a “1” and “0” debossed on the other side | score on one side and either a “2” and “0” debossed on the other side | |
| Score | Functional score (2 pieces) | Functional score (2 pieces) | |
| Color | a white to off-white | a white to off-white | |
| Shape | OVAL | OVAL | |
| Dimension | 9mm | 11mm | |
| Mfg by | Catalent Pharma Solutions (US) | ||
| Mfg for | Lundbeck (US) | ||
| Marketed by | - | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| There are no unexpired patents for this product in the Orange Book Database. | ||||||||
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.1 N HCl (degassed) | 900 | 5, 10, 20, 30, 45 and 60 | July 31, 2013 |
| II (Paddle) | 75 | 0.1 N HCl (degassed) | 900 | 10, 20, 30, 45 and 60 | October 31, 2013 |
| Market | EU | US |
|---|---|---|
| Strength | Packaging System | |
| 10MG | - | Bottles of 100 |
| 20MG | - | Bottles of 100 |
| Storage | Store tablets at 20°C to 25C (68°F to 77F). See USP controlled room temperature. | |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | AUDEN | Download |
| UK | AUDEN | Download |
| US | ONFI | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
