| Active Ingredient | BINIMETINIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEKTOVI | 210498 | ARRAY BIOPHARMA INC | TABLET;ORAL | 15MG | 15MG | June 27, 2018 | June 27, 2023 | June 27, 2025 | Type 1 - New Molecular Entity and Type 4 - New Combination | STANDARD; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
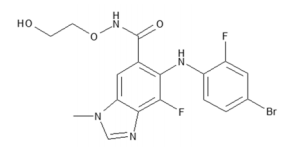 |
| Chemical Name | 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide |
| CAS No | 606143-89-9 |
| Molecular Formula | C17H15BrF2N4O3 |
| Molecular Weight | 441.2 daltons |
| Appearance | White to slightly yellow powder |
| Solubility | Slightly soluble at pH 1,very slightly soluble at pH 2, and practically insoluble at pH 4.5 and higher |
| Water Solubility | - |
| Polymorphism | Crystalline anhydrous Form |
| pKa (Strongest Acidic) | 2.5 |
| pKa (Strongest Basic) | - |
| Log P | 3.81 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | Class IV |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | MEKTOVI is a kinase inhibitor indicated, in combination with encorafenib,for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test. |
| Dosage and Administration |
Confirm the presence of BRAF V600E or V600K mutation in tumor specimens prior to the initiation of MEKTOVI. The recommended dose is 45 mg orally twice daily in combination with encorafenib. Take MEKTOVI with or without food. For patients with moderate or severe hepatic impairment the recommended dose is 30 mg orally twice daily |
| Mechanism of action |
Binimetinib is a reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models. Binimetinib and encorafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared to either drug alone, coadministration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of binimetinib and encorafenib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone. |
| Absorption |
The pharmacokinetics of binimetinib was studied in healthy subjects and patients with solid tumors. After twice-daily dosing, the accumulation is 1.5-fold and the coefficient of variation (CV%) of the area under the concentration-time curve (AUC) is < 40% at steady state. The systemic exposure of binimetinib is approximately dose proportional. Absorption After oral administration, at least 50% of the binimetinib dose was absorbed with a median time to maximum concentration (Tmax) of 1.6 hours. |
| Food Effect | The administration of a single dose of MEKTOVI 45 mg with a high-fat, high-calorie meal (consisting of approximately 150 calories from protein, 350 calories from carbohydrate, and 500 calories from fat) in healthy subjects had no effect on binimetinib exposure. |
| Distribution | Binimetinib is 97% bound to human plasma proteins and the blood-to-plasma ratio is 0.72. The geometric mean (CV%) of apparent volume of distribution of binimetinib is 92 L (45%). |
| Metabolism | The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib. |
| Elimination |
The mean (CV%) terminal half-life (t1/2) of binimetinib is 3.5 hours (28.5%) and apparent clearance (CL/F) is 20.2 L/h (24%). Excretion Following a single oral dose of 45 mg radiolabeled binimetinib in healthy subjects, 62% (32% unchanged) of the administered dose was recovered in the feces while 31% (6.5% unchanged) was recovered in the urine. |
| Peak plasma time (Tmax) | 1.6 hours |
| Half life | 3.5 hours (28.5%) |
| Bioavailability | 50% |
| Age, gender | Age (20 to 94 years), sex, or body weight do not have a clinically important effect on the systemic exposure of binimetinib. The effect of race or ethnicity on the pharmacokinetics of binimetinib is unknown. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 15 MG |
| Excipients used | Lactose monohydrate (133.5 mg), microcrystalline cellulose, croscarmellose sodium, magnesium stearate (vegetable source), and colloidal silicon dioxide |
| Composition of coating material | Polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc, ferric oxide yellow, and ferrosoferric oxide |
| Composition of caspule shell | - |
| Pharmaceutical Development | - |
| Manufacture of the product |
Comprising serial dilution / dry blending of drug substance with excipients, followed by compression, film-coating and packaging. |
| Tablet / Capsule Image | |
| Appearance | Yellow/dark yellow, unscored biconvex oval film-coated tablets debossed with a stylized “A” on one side and “15” on the other side |
| Imprint code / Engraving / Debossment | Debossed with a stylized “A” on one side and “15” on the other side |
| Score | Unscored |
| Color | Yellow/dark yellow |
| Shape | Oval |
| Dimension | 12 mm in length and 5 mm in width |
| Mfg by |
For EU: Pierre Fabre Médicament Production Aquitaine Pharm International 1 Avenue du Béarn 64320 Idron France |
| Mfg for | - |
| Marketed by |
For EU: Pierre Fabre Médicament 45, place Abel Gance 92100 Boulogne-Billancourt France |
| Distributed by |
Array BioPharma Inc. 3200 Walnut Street Boulder, CO 80301 |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N210498 | 1 | 10005761 | 27-08-2030 | - | - | U-2331 | - | Download |
| N210498 | 1 | 7777050 | 13-03-2023 | DS | DP | - | - | Download |
| N210498 | 1 | 8178693 | 13-03-2023 | DS | DP | - | - | Download |
| N210498 | 1 | 8193229 | 13-03-2023 | - | - | U-2330 | - | Download |
| N210498 | 1 | 8513293 | 13-03-2023 | - | - | U-2331 | - | Download |
| N210498 | 1 | 9314464 | 04-07-2031 | - | - | U-2332 | - | Download |
| N210498 | 1 | 9562016 | 18-10-2033 | DS | DP | - | - | Download |
| N210498 | 1 | 9593100 | 27-08-2030 | - | DP | - | - | Download |
| N210498 | 1 | 9598376 | 18-10-2033 | - | - | U-2330 | - | Download |
| N210498 | 1 | 9850229 | 27-08-2030 | - | - | U-2333 | - | Download |
| N210498 | 1 | 9980944 | 18-10-2033 | - | - | U-2334 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 RPM | 0.01 N HCl | 900 mL | Q point at 30 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | MEKTOVI | Download |
| UK | MEKTOVI | Download |
| US | MEKTOVI | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
