| Active Ingredient | BALOXAVIR MARBOXIL |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XOFLUZA | 210854 | SHIONOGI INC | TABLET;ORAL | 20 MG, 40 MG | 40 MG | October 24, 2018 | October 24, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
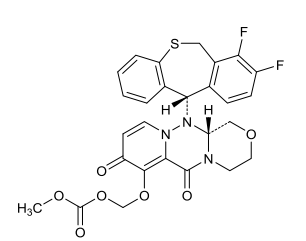 |
| Chemical Name | ({(12aR)-12-[(11S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin11-yl]-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl}oxy)methyl methyl carbonate |
| CAS No | 1985606-14-1 |
| Molecular Formula | C27H23F2N3O7S |
| Molecular Weight | 571.55 |
| Appearance | - |
| Solubility | It is freely soluble in dimethylsulfoxide, soluble in acetonitrile, slightly soluble in methanol and ethanol |
| Water Solubility | Practically insoluble in water |
| Polymorphism | - |
| pKa (Strongest Acidic) | - |
| pKa (Strongest Basic) | - |
| Log P | 2.26 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | XOFLUZATM is a polymerase acidic (PA) endonuclease inhibitor indicated for the treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 hours. Limitations of Use: Influenza viruses change over time, and factors such as the virus type or subtype, emergence of resistance, or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating influenza virus strains when deciding whether to use XOFLUZA. |
| Dosage and Administration |
Take a single dose of XOFLUZA orally within 48 hours of symptom onset with or without food. Avoid co-administration of XOFLUZA with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids, or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc). The dose of XOFLUZA depends on weight. 40 kg to less than 80 kg Body weight: Single dose of 40 mg At least 80 kg Body weight: Single dose of 80 mg |
| Mechanism of action |
Baloxavir marboxil is a prodrug that is converted by hydrolysis to baloxavir, the active form that exerts antiinfluenza virus activity. Baloxavir inhibits the endonuclease activity of the polymerase acidic (PA) protein, an influenza virus-specific enzyme in the viral RNA polymerase complex required for viral gene transcription, resulting in inhibition of influenza virus replication. The 50% inhibitory concentration (IC50) of baloxavir was 1.4 to 3.1 nM (n=4) for influenza A viruses and 4.5 to 8.9 nM (n=3) for influenza B viruses in a PA endonuclease assay. Viruses with reduced susceptibility to baloxavir have amino acid substitutions in the PA protein |
| Absorption | Baloxavir marboxil is a prodrug that is almost completely converted to its active metabolite, baloxavir,following oral administration.In the phase 3 trial, at the recommended dose of 40 mg for subjects weighing less than 80 kg, the mean (CV%) values of baloxavir Cmax and AUC0-inf were 96.4 ng/mL (45.9%) and 6160 ng·hr/mL (39.2%), respectively. At the recommended dose of 80 mg for subjects weighing 80 kg and more, the mean (CV%) values of baloxavir Cmax and AUC0-inf were 107 ng/mL (47.2%) and 8009 ng·hr/mL (42.4%), respectively. |
| Food Effect | Cmax: ↓48%, AUC0-inf; ↓36% (Meal: approximately 400 to 500 kcal including 150 kcal from fat ) |
| Distribution |
% Bound to human serum proteins: 92.9 - 93.9 Ratio of blood cell to blood: 48.5% - 54.4% Volume of distribution (V/F, L): 1180 (20.8%) |
| Metabolism | Metabolic pathways: UGT1A3, CYP3A4 |
| Elimination |
Major route of elimination : Metabolism Clearance (CL/F, L/hr): 10.3 (22.5%) |
| Peak plasma time (Tmax) | 4 Hours |
| Half life | 79.1 (22.4%) Hours |
| Bioavailability | - |
| Age, gender | There were no clinically significant differences in the pharmacokinetics of baloxavir based on age (adolescents as compared to adults), or sex. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||
|---|---|---|---|
| Strength | 20 MG | 40 MG | |
| Excipients used |
Croscarmellose sodium,lactose monohydrate, microcrystalline cellulose, povidone, sodium stearyl fumarate |
||
| Composition of coating material | Hypromellose, talc, and titanium dioxide | ||
| Composition of caspule shell | - | ||
| Pharmaceutical Development | - | ||
| Manufacture of the product | - | ||
| Tablet / Capsule Image | |||
| Appearance | White to light yellow, oblong shaped film-coated tablets debossed with “ 772” on one side and “20” on the other side | White to light yellow, oblong shaped film-coated tablets debossed with “BXM40” on one side available | |
| Imprint code / Engraving / Debossment | Debossed with “ 772” on one side and “20” on the other side | Debossed with “BXM40” on one side available | |
| Score | No score | No score | |
| Color | White to light yellow | White to light yellow | |
| Shape | Oblong shaped | Oblong shaped | |
| Dimension | 9 mm | 11 mm | |
| Mfg by |
Shionogi & Co., Ltd. 2-5-1 Mishima, Settsu Osaka 566-0022, Japan |
||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by |
Genentech USA, Inc. A Member of the Roche Group 1 DNA Way South San Francisco, CA 94080-4990 |
||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N210854 | 1 | 8927710 | May 5, 2031 | - | DP | - | - | Download |
| N210854 | 1 | 8987441 | September 21, 2031 | DS | DP | - | - | Download |
| N210854 | 1 | 9815835 | June 14, 2030 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 RPM | 0.07%w/v (for 20 mg) or 0.16% w/v (for 40 mg) CTAB in phosphate buffer, pH 6.8 | 900 mL | Q point in 30 min | as per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
