| Active Ingredient | AXITINIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INLYTA | (NDA) 202324 | PFIZER | TABLET;ORAL | 1MG, 5MG | 5MG (RS) | January 27, 2012 | January 27, 2017 | - | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
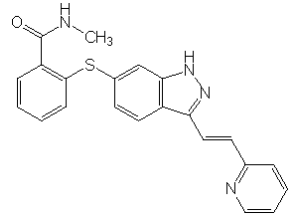 |
| Chemical Name | N-methyl-2-[3-((E)2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide |
| CAS No | 319460-85-0 |
| Molecular Formula | C22 H18 N4OS |
| Molecular Weight | 386.47 Daltons |
| Appearance | a white to light-yellow powder |
| Solubility | The solubility of axitinib in aqueous media over the range pH 1.1 to pH 7.8 is in excess of 0.2 µg/mL. |
| Water Solubility | The solubility of axitinib in aqueous media over the range pH 1.1 to pH 7.8 is in excess of 0.2 µg/mL. |
| Polymorphism | Five crystalline anhydrous forms have been identified (Form I, Form IV, Form VI, Form XXV and Form XLI). A number of crystalline solvates and hydrate forms have been observed and an amorphous form has been prepared. The polymorphic form intended for marketing is Form XLI. |
| pKa (Strongest Acidic) | 4.8 |
| pKa (Strongest Basic) | - |
| Log P | 3.5 |
| Identification | Infrared (IR) and by High Performance Liquid Chromatography (HPLC) |
| Degradation | - |
| Hygroscopic | non-hygrospic |
| Photostability study | Light sensitive (Photo labile) |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | The active substance is manufactured in five synthetic steps followed by recrystallisation. A design space has been developed for steps and milling and operational boundaries are proposed for all process parameters. Both the polymorphic form and the particle size distribution are critical quality attributes (CQA) for the dissolution of the active substance, which is classified as BCS class II drug (low solubility, high permeability). Both the polymorphic form and the particle size distribution are limited in the active substance specification. |
| Parameters | Details |
|---|---|
| Indications and Usage | INLYTA is indicated for the treatment of advanced renal cell carcinoma (RCC) after failure of one prior systemic therapy. |
| Dosage and Administration |
The recommended starting oral dose of INLYTA is 5 mg twice daily. Administer INLYTA doses approximately 12 hours apart with or without food. INLYTA should be swallowed whole with a glass of water. If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time. |
| Mechanism of action | Axitinib has been shown to inhibit receptor tyrosine kinases including vascular endothelial growth factor receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 at therapeutic plasma concentrations. These receptors are implicated in pathologic angiogenesis, tumor growth, and cancer progression. VEGFmediated endothelial cell proliferation and survival were inhibited by axitinib in vitro and in mouse models. Axitinib was shown to inhibit tumor growth and phosphorylation of VEGFR-2 in tumor xenograft mouse models. |
| Absorption |
The population pharmacokinetic analysis pooled data from 17 trials in healthy subjects and patients with cancer. A two-compartment disposition model with first-order absorption and lag-time adequately describes the axitinib concentration-time profile. Following single oral 5-mg dose administration, the median Tmax ranged from 2.5 to 4.1 hours. Based on the plasma half-life, steady state is expected within 2 to 3 days of dosing. Dosing of axitinib at 5 mg twice daily resulted in approximately 1.4-fold accumulation compared to administration of a single dose. At steady state, axitinib exhibits approximately linear pharmacokinetics within the 1-mg to 20-mg dose range. The mean absolute bioavailability of axitinib after an oral 5 mg dose is 58%. |
| Food Effect | Compared to overnight fasting, administration of INLYTA with a moderate fat meal resulted in 10% lower AUC and a high fat, high-calorie meal resulted in 19% higher AUC. INLYTA can be administered with or without food. |
| Distribution | Axitinib is highly bound (>99%) to human plasma proteins with preferential binding to albumin and moderate binding to α1-acid glycoprotein. In patients with advanced RCC (n=20), at the 5 mg twice daily dose in the fed state, the geometric mean (CV%) C max and AUC 0-24 were 27.8 (79%) ng/mL and 265 (77%) ng.h/mL, respectively. The geometric mean (CV%) clearance and apparent volume of distribution were 38 (80%) L/h and 160 (105%) L, respectively. |
| Metabolism | The plasma half life of INLYTA ranges from 2.5 to 6.1 hours. Axitinib is metabolized primarily in the liver by CYP3A4/5 and to a lesser extent by CYP1A2, CYP2C19, and UGT1A1. |
| Elimination |
Following oral administration of a 5-mg radioactive dose of axitinib, approximately 41% of the radioactivity was recovered in feces and approximately 23% was recovered in urine. Unchanged axitinib, accounting for 12% of the dose, was the major component identified in feces. Unchanged axitinib was not detected in urine; the carboxylic acid and sulfoxide metabolites accounted for the majority of radioactivity in urine. In plasma, the N-glucuronide metabolite represented the predominant radioactive component (50% of circulating radioactivity) and unchanged axitinib and the sulfoxide metabolite each accounted for approximately 20% of the circulating radioactivity. The sulfoxide and N-glucuronide metabolites show approximately ≥400-fold less in vitro potency against VEGFR-2 compared to axitinib. |
| Peak plasma time (Tmax) | 2.5 to 4.1 hours |
| Half life | 2.5 to 6.1 hours |
| Bioavailability | 58% |
| Age, gender | Population pharmacokinetic analyses indicate that there are no clinically relevant effects of age, gender, race, body weight, body surface area, UGT1A1 genotype, or CYP2C19 genotype on the clearance of axitinib. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 29672 | A | II | September 29, 2015 | MSN LABORATORIES PRIVATE LTD |
| 29918 | A | II | August 24, 2015 | ZHEJIANG JIUZHOU PHARMACEUTICAL CO LTD |
| 30104 | A | II | December 24, 2015 | SHILPA MEDICARE LTD |
| 30341 | A | II | March 4, 2016 | MSN LABORATORIES PRIVATE LTD |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 1MG | 5MG | |
| Excipients used | microcrystalline cellulose (63.25MG), lactose monohydrate (32MG), croscarmellose sodium (3MG), magnesium stearate (0.75MG) | microcrystalline cellulose (107.43MG), lactose monohydrate (56MG), croscarmellose sodium (5.25MG), magnesium stearate (1.32MG) | |
| Composition of coating material | The Opadry II red (4MG) 32K15441 film coating contains lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide. | The Opadry II red (7MG) 32K15441 film coating contains lactose monohydrate, HPMC 2910/Hypromellose 15cP, titanium dioxide, triacetin (glycerol triacetate), and red iron oxide. | |
| Composition of caspule shell | - | ||
| Pharmaceutical Development |
Polymorphic form XLI was chosen as it is the most thermodynamically stable polymorphic form. The excipients used in Inlyta are microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate and film coating Opadry II red (hypromellose, titanium dioxide, lactose monohydrate, triacetin and red iron oxide). The hypromellose based coating system is more compatible with axitinib. The purposeful degradation test demonstrated that the polymorphic form change and modification in coating system resulted in a chemically more stable formulation. Tablet was manufactured by dry granulation formulation to reduce processing time and material costs. A design space was developed for the manufacturing process. The critical quality attributes identified were uniformity of dosage units, dissolution and photo-stability. |
||
| Manufacture of the product |
Inlyta 1 and 5mg tablets are manufactured by a dry granulation process. The manufacturing process consists in blending, milling, dry granulation, tabletting, film-coating and packaging. A design space was developed for the milling, dry granulation and milling and the film coating steps of the manufacturing process and the critical quality attributes identified were uniformity of dosage units, dissolution and photo-stability. |
||
| Tablet / Capsule Image |
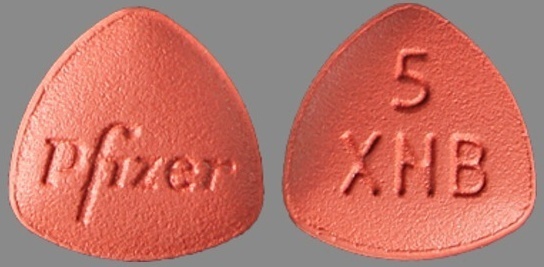
|
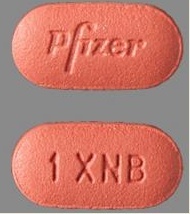
|
|
| Appearance | red, film-coated, oval tablets, debossed with “Pfizer” on one side and “1 XNB” on the other side | red, film-coated, triangular tablets, debossed with “Pfizer” on one side and “5 XNB” on the other side. | |
| Imprint code / Engraving / Debossment | debossed with “Pfizer” on one side and “1 XNB” on the other side | debossed with “Pfizer” on one side and “5 XNB” on the other side | |
| Score | no score | no score | |
| Color | RED | RED | |
| Shape | OVAL | TRIANGLE | |
| Dimension | 9mm | 8mm | |
| Mfg by | Pfizer Lab (EU) | ||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by | Pfizer Lab (US, EU) | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N202324 | 1 | 7141581 | June 30, 2020 | - | - | U - 1220 | - | Download |
| N202324 | 1 | 8791140 | August 5, 2030 | DS | - | - | - | Download |
| N202324 | - | 6534524 | April 29, 2025 | DS | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.01 N HCl | 900 | 5, 15, 30, 45 and 60 | August 14, 2014 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | INLYTA | Download |
| UK | INLYTA | Download |
| US | INLYTA | Download |
| Inlyta 3MG and 7MG tablet strength are additionaly approved in EU and UK. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
