| Active Ingredient | AVACOPAN |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAVNEOS | 214487 | CHEMOCENTRYX INC | CAPSULE;ORAL | 10MG | 10MG | October 7, 2021 | October 7, 2026 | _ | Type 1 - New Molecular Entity | STANDARD; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
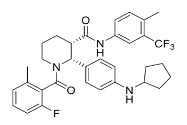 |
| Chemical Name | (2R,3S)-2-[4(cyclopentylamino)phenyl]-1-(2-fluoro-6-methylbenzoyl)-N-[4-methyl-3(trifluoromethyl)phenyl]piperidine-3-carboxamide. |
| CAS No | 1346623-17-3 |
| Molecular Formula | C33H35F4N3O2 |
| Molecular Weight | 582 g/mol |
| Appearance | White to pale yellow crystalline solid |
| Solubility | It is soluble in organic solvents and practically insoluble in water. |
| Water Solubility | 0.000219 mg/Ml |
| Polymorphism | - |
| pKa (Strongest Acidic) | 14.05 |
| pKa (Strongest Basic) | 4.98 |
| Log P | 6.82, 7.59 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | BCS Class II |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | TAVNEOS is a complement 5a receptor (C5aR) antagonist indicated as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. TAVNEOS does not eliminate glucocorticoid use. |
| Dosage and Administration | The recommended dosage is 30 mg (three 10 mg capsules) twice daily, with food. |
| Mechanism of action | Avacopan is a complement 5a receptor (C5aR) antagonist that inhibits the interaction between C5aR and the anaphylatoxin C5a. Avacopan blocks C5a-mediated neutrophil activation and migration. The precise mechanism by which avacopan exerts a therapeutic effect in patients with ANCA-associated vasculitis has not been definitively established. |
| Absorption |
Based on population pharmacokinetic analysis, the mean steady state plasma exposure estimates of avacopan are 3466 ± 1921 ngh/mL for the 12-hour area under the plasma drug concentration over time curve (AUC0-12hr) and 349 ± 169 ng/mL for the maximum plasma concentration (Cmax) in patients with ANCA-associated vasculitis receiving 30 mg avacopan twice daily. Steady state plasma levels of avacopan are reached by 13 weeks and the accumulation is approximately 4 fold. Absorption: Co-administration of 30 mg in capsule formulation with a high-fat, high-calorie meal increases AUC and Cmax of avacopan by approximately 72% and 8%, respectively, and delays tmax by approximately 4 hours (from 2.0 hours to 6.0 hours). |
| Food Effect | Co-administration of 30 mg in capsule formulation with a high-fat, high-calorie meal increases AUC and Cmax of avacopan by approximately 72% and 8%, respectively, and delays tmax by approximately 4 hours (from 2.0 hours to 6.0 hours). |
| Distribution | The plasma protein binding (e.g., to albumin and α1-acid glycoprotein) of avacopan and metabolite M1 is greater than 99.9%. The apparent volume of distribution of avacopan is estimated to be 345 L. |
| Metabolism | CYP3A4 is the major enzyme responsible for the clearance of avacopan and for the formation and clearance of the major circulating metabolite M1, a mono-hydroxylated product of avacopan. M1 was present at ~12% of the total drug-related materials in plasma and has approximately the same activity as avacopan on the C5aR. |
| Elimination |
Elimination : Based on population pharmacokinetic analysis, the estimated total apparent body clearance (CL/F) of avacopan is 16.3 L/h. Following a single dose of 30 mg avacopan with food, the mean elimination half-lives of avacopan and M1 are 97.6 hours and 55.6 hours, respectively, in healthy subjects. Excretion : The main route of clearance of avacopan is metabolism followed by biliary excretion of the metabolites into feces. Following oral administration of radiolabelled avacopan, about 77% and 10% of the radioactivity was recovered in feces and urine, respectively, and 7% and <0.1% of the radioactive dose was recovered as unchanged avacopan in feces and urine, respectively. |
| Peak plasma time (Tmax) | 4 hours (from 2.0 hours to 6.0 hours) |
| Half life | 97.6 hours |
| Bioavailability | - |
| Age, gender | No clinically significant differences in plasma exposure of avacopan and metabolite M1 were observed based on race (White, Asian, Black), gender (female 31%), age (18 to 83 years), body weight (40.3-174 kg), and renal function (eGFR 14-170 mL/min/1.73m2 at baseline). |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 10MG |
| Excipients used | Polyethylene glycol 4000 (PEG-4000), Polyoxyl-40 hydrogenated castor oil. |
| Composition of coating material | NA |
| Composition of caspule shell | Gelatin, red iron oxide, yellow iron oxide, and titanium dioxide, and the capsule sealing band contains gelatin and polysorbate 80 |
| Pharmaceutical Development | To be updated soon |
| Manufacture of the product | To be updated soon |
| Tablet / Capsule Image | |
| Appearance | Light orange and yellow opaque bicolor gelatin capsule with a clear gelatin sealing. capsule with “CCX168” printed in black. |
| Imprint code / Engraving / Debossment | Capsule with “CCX168” printed in black. |
| Score | No score |
| Color | ORANGE (light orange opaque) , YELLOW (yellow opaque) |
| Shape | CAPSULE |
| Dimension | 22mm |
| Mfg by | Thermo Fisher Scientific 2110 East Galbraith Road Cincinnati, OH 45237 USA |
| Mfg for | ChemoCentryx, Inc. |
| Marketed by | - |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N214487 | 1 | 8445515 | February 3,2031 | DS | DP | - | - | Download |
| N214487 | 1 | 8906938 | December 21, 2029 | DS | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| Apparatus II with sinker | 50 | 0.1N HCl/37°C±0.5°C | 900 | Q= % In 30 minutes | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
