| Active Ingredient | ATOMOXETINE HYDROCHLORIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STRATTERA | (NDA) 021411 | LILLY | CAPSULE;ORAL | 10 MG, 18 MG, 25 MG, 40 MG, 60 MG, 80 MG, 100 MG | 60 MG | November 26, 2002 | _ | _ | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
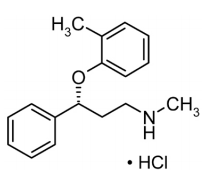 |
| Chemical Name | (-)-N-Methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride |
| CAS No | 83015-26-3 |
| Molecular Formula | C17H21NO•HCl |
| Molecular Weight | 291.82 |
| Appearance | White to practically white solid |
| Solubility | - |
| Water Solubility | 27.8 mg/mL |
| Polymorphism | - |
| pKa (Strongest Acidic) | - |
| pKa (Strongest Basic) | 9.8 (Predicted) |
| Log P | 3.9 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | STRATTERA®is a selective norepinephrine reuptake inhibitor indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). |
| Dosage and Administration | Dosing adjustment — Hepatic Impairment, Strong CYP2D6 Inhibitor, and in patients known to be CYP2D6 poor metabolizers (PMs). (Refer PIL) |
| Mechanism of action | The precise mechanism by which atomoxetine produces its therapeutic effects in Attention-Deficit/Hyperactivity Disorder (ADHD) is unknown, but is thought to be related to selective inhibition of the pre-synaptic norepinephrine transporter, as determined in ex vivo uptake and neurotransmitter depletion studies. |
| Absorption | Atomoxetine is rapidly absorbed after oral administration, with absolute bioavailability of about 63% in EMs and 94% in PMs. Maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing. |
| Food Effect | STRATTERA can be administered with or without food. Administration of STRATTERA with a standard high-fat meal in adults did not affect the extent of oral absorption ofatomoxetine (AUC), but did decrease the rate of absorption, resulting in a 37% lower Cmax, and delayed Tmaxby 3 hours. In clinical trials withchildren and adolescents, administration of STRATTERA with food resulted in a 9% lower Cmax. |
| Distribution | The steady-state volume of distribution after intravenous administration is 0.85 L/kg indicating that atomoxetine distributes primarily into total body water. Volume of distribution is similar across the patient weight range after normalizing for body weight. At therapeutic concentrations, 98% of atomoxetine in plasma is bound to protein, primarily albumin. |
| Metabolism |
Atomoxetine is metabolized primarily through the CYP2D6 enzymatic pathway. People with reduced activity in this pathway (PMs) have higher plasma concentrations of atomoxetine compared with people with normal activity (EMs). For PMs, AUC of tomoxetine is approximately 10-fold and Css, maxis about 5-fold greater than EMs. Laboratory tests are available to identify CYP2D6 PMs. Coadministration of STRATTERA with potent inhibitors of CYP2D6, such as fluoxetine, paroxetine, or quinidine, results in a substantial increase in atomoxetine plasma exposure, and dosing adjustment may be necessary [see Warnings and Precautions (5.13)]. Atomoxetine did not inhibit or induce the CYP2D6 pathway. The major oxidative metabolite formed,regardless of CYP2D6 status, is4-hydroxyatomoxetine, which is glucuronidated. 4-Hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter but circulates in plasma at much lower concentrations (1% ofatomoxetine concentration in EMs and 0.1% of atomoxetine concentration in PMs). 4-Hydroxyatomoxetine is primarily formed by CYP2D6, but in PMs, 4-hydroxyatomoxetine is formed at a slower rate by several other cytochrome P450 enzymes. N-Desmethylatomoxetine is formed by CYP2C19 and other cytochrome P450 enzymes, but has substantially less pharmacological activity compared with atomoxetine and circulates in plasma at lower concentrations (5% ofatomoxetine concentration in EMs and 45% of atomoxetine concentration in PMs). |
| Elimination |
Mean apparent plasma clearance of atomoxetine after oral administration in adult EMs is 0.35 L/hr/kg and the mean half-life is 5.2 hours. Following oral administration of atomoxetine to PMs, mean apparent plasma clearance is 0.03L/hr/kg and mean half-life is 21.6 hours. For PMs, AUC of atomoxetine is approximately 10-fold and Css, maxis about 5-fold greater than EMs. The elimination half-life of 4-hydroxyatomoxetine is similar to that of N-desmethylatomoxetine (6 to 8 hours) in EM subjects, while the half-life of N-desmethylatomoxetine is much longer in PM subjects (34 to 40 hours). Atomoxetine is excreted primarily as 4-hydroxyatomoxetine-O-glucuronide, mainly in the urine (greater than 80% of the dose) and to a lesser extent in the feces (less than 17% of the dose). Only a small fraction of the STRATTERA dose is excreted as unchanged atomoxetine (less than 3% of the dose), indicating extensive biotransformation. |
| Peak plasma time (Tmax) | 1 to 2 hours |
| Half life | 5.2 hours |
| Bioavailability | 63% in EMs and 94% in PMs |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 18440 | A | II | June 22, 2005 | DR REDDYS LABORATORIES LTD |
| 19605 | A | II | March 16, 2007 | AUROBINDO PHARMA LTD |
| 19623 | I | II | July 10, 2006 | OLON SPA |
| 19861 | A | II | October 12, 2006 | MYLAN LABORATORIES LTD |
| 20070 | A | II | May 18, 2007 | APOTEX PHARMACHEM INC |
| 20208 | I | II | January 18, 2007 | ZHEJIANG JIUZHOU PHARMACEUTICAL CO LTD |
| 20246 | A | II | February 7, 2007 | HETERO LABS LTD |
| 20499 | A | II | April 30, 2007 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 20552 | A | II | May 21, 2007 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 22862 | A | II | August 11, 2009 | CADILA PHARMACEUTICALS LTD |
| 29211 | A | II | March 26, 2015 | ZCL CHEMICALS LTD |
| 29868 | A | II | October 31, 2015 | MSN ORGANICS PRIVATE LTD |
| Parameters | Details | |||||||
|---|---|---|---|---|---|---|---|---|
| Strength | 25 MG | 60 MG | 40 MG | 18 MG | 80 MG | 100 MG | 10 MG | |
| Excipients used | Pregelatinized starch and dimethicone | |||||||
| Composition of coating material | - | |||||||
| Composition of caspule shell |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: FD&C Blue 2 (Indigo Carmine) E132 and Titanium dioxide E 171 Capsule Shell Body colourants: Titanium dioxide E 171 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: FD&C Blue 2 (Indigo Carmine) E132 and Titanium dioxide E 171 Capsule Shell Body colourants:Yellow iron oxide E172 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: FD&C Blue 2 (Indigo Carmine) E132 and Titanium dioxide E 171 Capsule Shell Body colourants:FD&C Blue 2 (Indigo Carmine) E132 and Titanium dioxide E 171 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: Yellow iron oxide E172 Capsule Shell Body colourants: Titanium dioxide E 171 |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: Yellow iron oxide E172, Red iron oxide E172, Titanium dioxide E171 Capsule Shell Body colourants: Titanium dioxide E 171 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: Yellow iron oxide E172, Red iron oxide E172, Titanium dioxide E171 Capsule Shell Body colourants:Yellow iron oxide E172, Red iron oxide E172, Titanium dioxide E171 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
Gelatin, sodium lauryl sulfate Capsule shell cap colourants: Titanium dioxide E 171 Capsule Shell Body colourants: Titanium dioxide E 171 Edible Black Ink SW-9008 (containing Shellac and Black Iron Oxide E172) or Edible Black Ink SW-9010 (containing Shellac and Black Iron Oxide E172). |
|
| Pharmaceutical Development | - | |||||||
| Manufacture of the product | - | |||||||
| Tablet / Capsule Image |
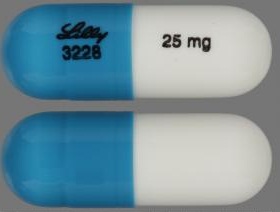
|

|
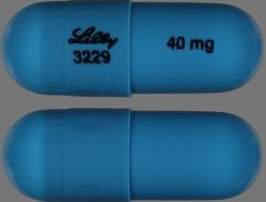
|

|
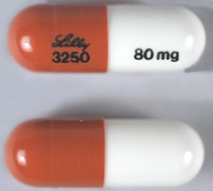
|
|

|
|
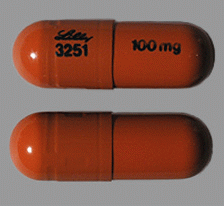
| Appearance | Opaque Blue, Opaque White Identification: LILLY 3228 and 25 mg | Opaque Blue, Gold identification: LILLY 3239 and 60 mg | Opaque Blue, Opaque Blue identification: LILLY 3229 and 40 mg | Gold, Opaque White Identification: LILLY 3238 and 18 mg | Opaque Brown, Opaque White identification: LILLY 3250 and 80 mg | Opaque Brown, Opaque Brown identification: LILLY 3251 and 100 mg | Opaque White, Opaque White. Identification: LILLY 3227 and 10 mg | |
| Imprint code / Engraving / Debossment | Identification: LILLY 3228 and 25 mg | identification: LILLY 3239 and 60 mg | identification: LILLY 3229 and 40 mg | Identification: LILLY 3238 and 18 m | identification: LILLY 3250 and 80 mg | identification: LILLY 3251 and 100 mg | Identification: LILLY 3227 and 10 mg | |
| Score | No score | No score | No score | No score | No score | No score | No score | |
| Color | Opaque Blue, Opaque White | Opaque Blue, Gold | Opaque Blue, Opaque Blue | Gold, Opaque White | Opaque Brown, Opaque White | Opaque Brown, Opaque Brown | Opaque White, Opaque White. | |
| Shape | Capsule | Capsule | Capsule | Capsule | Capsule | Capsule | Capsule | |
| Dimension | 15.5-16.1 mm length | 17.5-18.1 mm length | 15.5-16.1 mm length | 15.5-16.1 mm length | 17.5-18.1 mm length | 19.2-19.8 mm length | 15.5-16.1 mm length | |
| Mfg by | - | |||||||
| Mfg for | - | |||||||
| Marketed by | Lilly USA, LLC | |||||||
| Distributed by | - | |||||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021411 | 2 | 5658590 | November 26, 2016 | - | - | U - 494 | - | Download |
| N021411 | 2 | 5658590*PED | May 26, 2017 | - | - | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.1 N HCl | 1000 | 10, 20, 30 and 45 | December 20, 2005 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | Strattera | Download |
| UK | Strattera | Download |
| US | APOTEX INC*(Tentative Approval) | |
| US | AUROBINDO PHARMA USA*(Tentative Approval) | |
| US | DR REDDYS LABS LTD*(Tentative Approval) | |
| US | GLENMARK GENERICS*(Tentative Approval) | |
| US | MYLAN PHARMS INC*(Tentative Approval) | |
| US | SANDOZ INC*(Tentative Approval) | |
| US | Strattera | Download |
| US | SUN PHARMA GLOBAL*(Tentative Approval) |
| Date of first authorisation in EU: 27 May 2004, Date of last renewal in EU: 27 May 2014. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
