| Active Ingredient | APIXABAN |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELIQUIS | (NDA) 202155 | BRISTOL MYERS SQUIBB | TABLET;ORAL | 2.5MG, 5MG | 5MG (RS) | December 28, 2012 | December 28, 2017 | - | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
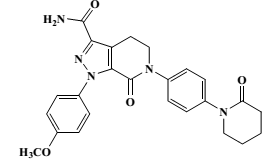 |
| Chemical Name | 1-(4methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4c]pyridine-3-carboxamide |
| CAS No | 503612-47-3 |
| Molecular Formula | C25H25N5O4 |
| Molecular Weight | 459.5 |
| Appearance | a white to pale yellow, crystalline powder |
| Solubility | At physiological pH (1.2–6.8), apixaban does not ionize; its aqueous solubility across the physiological pH range is 0.04 mg/mL |
| Water Solubility | 0.0679 mg/mL |
| Polymorphism | It shows polymorphism and a number of hydrates and solvates were identified. However, only one form is consistently produced by the proposed synthetic process. |
| pKa (Strongest Acidic) | 13.12 (Predicted) |
| pKa (Strongest Basic) | - |
| Log P | 1.65 |
| Identification | Raman/IR, HPLC |
| Degradation | - |
| Hygroscopic | non hygroscopic |
| Photostability study | - |
| Melting Point | 238-240°C |
| BCS Class | III |
| Manufacture of API | The commercial manufacturing process for the synthesis of apixaban involves a sequence of five reactions with two isolated intermediates. The quality by design (QbD) concept has been used during the development of the manufacturing process for Apixaban drug substance.The critical quality attributes (CQAs) of the drug substance were identified on the basis of their potential impact on the safety and efficacy of the drug product and thus to the patient. |
| Parameters | Details |
|---|---|
| Indications and Usage | ELIQUIS (apixaban) is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. ELIQUIS is indicated for the prophylaxis of deepvein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who haveundergone hip or knee replacement surgery. ELIQUIS is indicated for the treatment pulmonary embolism (PE). ELIQUIS is indicated to reduce the risk of recurrent DVT and PE following initial therapy. |
| Dosage and Administration |
Reduction of Risk of Stroke and Systemic Embolism in Patients with Nonvalvular Atrial Fibrillation:The recommended dose of ELIQUIS for most patients is 5 mg taken orally twice daily. Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery: The recommended dose of ELIQUIS is 2.5 mg taken orally twice daily. The initial dose should be taken 12 to 24 hours after surgery. In patients undergoing hip replacement surgery, the recommended duration of treatment is 35 days. In patients undergoing knee replacement surgery, the recommended duration of treatment is 12 days. Treatment of DVT and PE: The recommended dose of ELIQUIS is 10 mg taken orally twice daily for the first 7 days of therapy. After 7 days, the recommended dose is 5 mg taken orally twice daily. Reduction in the Risk of Recurrence of DVT and PE: The recommended dose of ELIQUIS is 2.5 mg taken orally twice daily after at least 6 months of treatment for DVT or PE. |
| Mechanism of action | Apixaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Apixaban inhibits free and clot-bound FXa, and prothrombinase activity. Apixaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, apixaban decreases thrombin generation and thrombus development. |
| Absorption |
Apixaban demonstrates linear pharmacokinetics with dose-proportional increases in exposure for oral doses up to 10 mg. The absolute bioavailability of apixaban is approximately 50% for doses up to 10 mg of ELIQUIS.Maximum concentrations (Cmax) of apixaban appear 3 to 4 hours after oral administration of ELIQUIS. At doses ≥25 mg, apixaban displays dissolution-limited absorption with decreased bioavailability. Following administration of a crushed 5 mg ELIQUIS tablet that was suspended in 60 mL D5W and delivered through a nasogastric tube (NGT), exposure was similar to that seen in other clinical trials involving healthy volunteers receiving a single oral 5 mg tablet dose. |
| Food Effect | Food does not affect the bioavailability of apixaban. |
| Distribution | Plasma protein binding in humans is approximately 87%. The volume of distribution (Vss) is approximately 21 liters. |
| Metabolism |
Approximately 25% of an orally administered apixaban dose is recovered in urine and feces as metabolites. Apixaban is metabolized mainly via CYP3A4 with minor contributions from CYP1A2, 2C8, 2C9, 2C19, and 2J2. O-demethylation and hydroxylation at the 3-oxopiperidinyl moiety are the major sites of biotransformation. Unchanged apixaban is the major drug-related component in human plasma; there are no active circulating metabolites. |
| Elimination |
Apixaban is eliminated in both urine and feces. Renal excretion accounts for about 27% of total clearance. Biliary and direct intestinal excretion contributes to elimination of apixaban in the feces. Apixaban has a total clearance of approximately 3.3 L/hour and an apparent half-life of approximately 12 hours following oral administration. Apixaban is a substrate of transport proteins: P-gp and breast cancer resistance protein. |
| Peak plasma time (Tmax) | 3 to 4 hours |
| Half life | 12 hours |
| Bioavailability | 50% for doses up to 10 mg of ELIQUIS |
| Age, gender | A study in healthy subjects comparing the pharmacokinetics in males and females showed no meaningful difference. No dose adjustment is required for different age group people. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 28009 | A | II | February 26, 2014 | MSN LABORATORIES PRIVATE LTD |
| 29660 | A | II | September 30, 2015 | ALEMBIC PHARMACEUTICALS LTD |
| 29667 | A | II | February 9, 2015 | ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD |
| 29707 | A | II | September 22, 2015 | DR REDDYS LABORATORIES LTD |
| 30060 | A | II | December 31, 2015 | GLENMARK PHARMACEUTICALS LTD |
| 30336 | A | II | March 14, 2016 | DELMAR CHEMICALS INC |
| 30440 | A | II | April 1, 2016 | SCINOPHARM TAIWAN LTD |
| 30461 | A | II | May 18, 2016 | HONOUR LAB LTD |
| 30479 | A | II | June 20, 2016 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 30613 | A | II | July 20, 2016 | INDOCO REMEDIES LTD |
| 30619 | A | II | August 12, 2016 | MICRO LABS LTD |
| 30630 | A | II | July 6, 2016 | CADILA HEALTHCARE LTD |
| 30635 | A | II | July 29, 2016 | WATSON PHARMA PRIVATE LTD |
| 30646 | A | II | June 30, 2016 | MEDICHEM MANUFACTURING MALTA LTD |
| 30653 | A | II | June 28, 2016 | SINTENOVO SA DE CV |
| 30704 | A | II | July 12, 2016 | AUROBINDO PHARMA LTD |
| 30774 | A | II | August 10, 2016 | SIGMAPHARM LABORATORIES LLC |
| 30788 | A | II | September 23, 2016 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 30845 | A | II | September 30, 2016 | NATCO PHARMA LTD |
| 30874 | A | II | August 31, 2016 | HEC PHARM CO LTD |
| 30929 | A | II | September 16, 2016 | UNICHEM LABORATORIES LTD |
| 31089 | A | II | October 31, 2016 | MSN LABORATORIES PRIVATE LTD |
| 31166 | A | II | November 16, 2016 | ZAKLADY FARMACEUTYCZNE POLPHARMA SA |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 2.5MG | 5MG | |
| Excipients used | anhydrous lactose (50.25MG), microcrystalline cellulose (41MG), croscarmellose sodium (4MG), sodium lauryl sulfate (1MG), and magnesium stearate (1.25MG) | anhydrous lactose (100.5MG), microcrystalline cellulose (82MG), croscarmellose sodium (8MG), sodium lauryl sulfate (2MG), and magnesium stearate (2.5MG) | |
| Composition of coating material | Opadry II yellow (4MG): lactose monohydrate, hypromellose, titanium dioxide, triacetin, and yellow iron oxide | Opadry II red (8MG): lactose monohydrate, hypromellose, titanium dioxide, triacetin, and red iron oxide | |
| Composition of caspule shell | - | ||
| Pharmaceutical Development |
The apixaban tablets were designed to produce tablets with rapid disintegration and dissolution characteristics ensuring consistent and acceptable bioavailability. Apixaban is non-ionisable, and therefore, its aqueous solubility is not affected by changes in pH. It is highly soluble for doses up to 10 mg and is a low permeable compound since the fraction of oral dose absorbed is < 90%. Thus, as per the Biopharmaceutical Classification System (BCS), it is classified as a Class III (high solubility/low permeability) compound. Both 2.5 and 5 mg tablets are manufactured from a common granulate. Development work included the evaluation of the different types of granulation and the use of a film coat for the tablets. It was found that tablets made using the dry granulation process have more consistent dissolution rates. Based on a relative bioavailability study results, the minimum dissolution requirement for apixaban was established. The film coat of the tablet is non-functional and has no impact on drug product performance. It has been optimised during development with regard to the aesthetic aspect. The particle size of the drug substance was identified as a critical factor based on its influence on the dissolution of the tablets. It was recognized that because of the targeted low dose for apixaban, content uniformity of the final product becomes an important product attribute Since the drug substance is mixed with most of the excipients at the 1st blending step (preblending), a quantitative on-line NIR method was developed to monitor the mixing profile during the preblending unitoperation, including the acceptance criteria for determining the blending endpoint. The proposed commercial manufacturing process uses process analytical technology (PAT), as well as conventional analytical tests, to control the in-process intermediates. |
||
| Manufacture of the product |
The manufacturing process comprises the following main steps: blending, granulation, blending, tabletting, film-coating and packaging. Due to the initially proposed use of NIR (as PAT) and RTR for the manufacturing of the drug product, the proposed finished product manufacturing site has been inspected in relation to this application during the clock-stop period. Responses to observations have been received and found to be mostly adequate. |
||
| Tablet / Capsule Image |
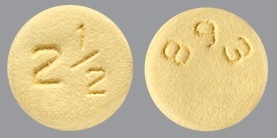
|

|
|
| Appearance | yellow, round, biconvex, film-coated tablets with “893” debossed on one side and “2½” on the other side | pink, oval-shaped, biconvex, film-coated tablets with “894” debossed on one side and “5” on the other side | |
| Imprint code / Engraving / Debossment | “893” debossed on one side and “2½” on the other side | “894” debossed on one side and “5” on the other side | |
| Score | no score | no score | |
| Color | YELLOW | PINK | |
| Shape | ROUND BICONVEX | OVAL BICONVEX | |
| Dimension | 6mm | 10mm | |
| Mfg by | Bristol-Myers Squibb Company (EU) | ||
| Mfg for | - | ||
| Marketed by | Bristol-Myers Squibb Company (US, EU) | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N202155 | 1 | 6413980 | December 22, 2019 | DS | DP | U-1200 U-1301 U-1302 U-1501 | - | Download |
| N202155 | 1 | 6967208 | September 17, 2022 | DS | DP | U-1167 U-1200 U-1301 U-1302 U-1323 U | - | Download |
| N202155 | 1 | 9326945 | February 24, 2031 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.05 M Sodium Phosphate Buffer with 0.05% SLS, pH 6.8 | 900 | 5, 10, 20, 30 and 45 | May 9, 2013 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | ELIQUIS | Download |
| UK | ELIQUIS | Download |
| US | ELIQUIS | Download |
| Only ELIQUIS 2.5MG tablet is approved in EU and UK. Exclusivity Code: Exclusivity Expiration; I-661: Aug 21, 2017; I-681 Mar 3, 2017; I-690: Aug 21, 2017; I-691: Aug 21, 2017 |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
