| Active Ingredient | ALFUZOSIN HYDROCHLORIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UROXATRAL | (NDA) 021287 | CONCORDIA PHARMS INC | TABLET, EXTENDED RELEASE;ORAL | 10MG | 10MG | June 12, 2003 | _ | _ | 1 New molecular entity (NME) | S Standard review drug | Prescription | AB |
| Parameters | Details |
|---|---|
| Structural Formula |
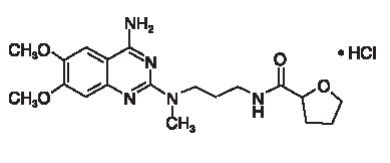 |
| Chemical Name | (R,S)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl) methylamino] propyl] tetrahydro-2-furancarboxamide hydrochloride |
| CAS No | 81403-80-7 |
| Molecular Formula | C19H27N5O4•HCl |
| Molecular Weight | 425.9 |
| Appearance | White to off-white crystalline powder |
| Solubility | Sparingly soluble in alcohol, and practically insoluble in dichloromethane |
| Water Solubility | Freely soluble in water, |
| Polymorphism | - |
| pKa (Strongest Acidic) | 14.64 (Predicted) |
| pKa (Strongest Basic) | 7.3 (Predicted) |
| Log P | 1.4 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 240°C |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | UROXATRAL is an alpha adrenergic antagonist, indicated for the treatment of signs and symptoms of benign prostatic hyperplasia. Important Limitations of Use: UROXATRAL is not indicated for treatment of hypertension. UROXATRAL is not indicated for use in the pediatric population. |
| Dosage and Administration |
10 mg once daily with food and with the same meal each day. Tablets should not be chewed or crushed |
| Mechanism of action | Alfuzosin is a selective antagonist of post-synaptic alpha1-adrenoreceptors, which are located in the prostate, bladder base, bladder neck, prostatic capsule, and prostatic urethra. |
| Absorption | The absolute bioavailability of UROXATRAL 10 mg tablets under fed conditions is 49%. Following multiple dosing of 10 mg UROXATRAL under fed conditions, the time to maximum concentration is 8 hours. Cmax and AUC0-24 are 13.6 (SD = 5.6) ng/mL and 194 (SD = 75) ng·h/mL, respectively. UROXATRAL exhibits linear kinetics following single and multiple dosing up to 30 mg. Steady-state plasma levels are reached with the second dose of UROXATRAL administration. Steady-state alfuzosin plasma concentrations are 1.2- to 1.6-fold higher than those observed after a single administration. |
| Food Effect | The extent of absorption is 50% lower under fasting conditions. Therefore, UROXATRAL should be taken with food and with the same meal each day |
| Distribution | The volume of distribution following intravenous administration in healthy male middle-aged volunteers was 3.2 L/kg. Results of in vitro studies indicate that alfuzosin is moderately bound to human plasma proteins (82% to 90%), with linear binding over a wide concentration range (5 to 5,000 ng/mL). |
| Metabolism | Alfuzosin undergoes extensive metabolism by the liver, with only 11% of the administered dose excreted unchanged in the urine. Alfuzosin is metabolized by three metabolic pathways: oxidation, O-demethylation, and N-dealkylation. The metabolites are not pharmacologically active. CYP3A4 is the principal hepatic enzyme isoform involved in its metabolism. |
| Elimination | Following oral administration of 14C-labeled alfuzosin solution, the recovery of radioactivity after 7 days (expressed as a percentage of the administered dose) was 69% in feces and 24% in urine. Following oral administration of UROXATRAL 10 mg tablets, the apparent elimination half-life is 10 hours. |
| Peak plasma time (Tmax) | 8 hours |
| Half life | 10 hours |
| Bioavailability | 49% |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 11976 | I | II | May 14, 1996 | SANOFI-SYNTHELABO INC |
| 12546 | I | II | June 3, 1997 | FINORGA |
| 17624 | A | II | August 24, 2004 | EXCELLA GMBH |
| 19180 | I | II | August 7, 2006 | CIPLA LTD |
| 19476 | A | II | May 26, 2006 | FARMAK AS |
| 19675 | A | II | August 10, 2006 | GLENMARK PHARMACEUTICALS LTD |
| 19786 | I | II | December 1, 2006 | STANDARD CHEM AND PHARM CO LTD |
| 19961 | I | II | November 14, 2006 | DR REDDYS LABORATORIES LTD |
| 20307 | A | II | February 24, 2007 | AUROBINDO PHARMA LTD |
| 20350 | I | II | March 13, 2007 | SHANDONG NEW TIME PHARMACEUTICAL CO LTD |
| 20379 | A | II | March 28, 2007 | CADILA HEALTHCARE LTD |
| 20439 | A | II | April 6, 2007 | WOCKHARDT LTD |
| 20540 | I | II | May 15, 2007 | LUNDBECK PHARMACEUTICALS ITALY SPA |
| 20577 | A | II | June 7, 2007 | HETERO DRUGS LTD |
| 20595 | A | II | June 8, 2007 | APOTEX PHARMACHEM INC |
| 20867 | A | II | September 17, 2007 | MSN LABORATORIES PRIVATE LTD |
| 20888 | A | II | September 27, 2007 | SANOFI CHIMIE SANOFI AVENTIS GROUP |
| 22638 | A | II | March 30, 2009 | UNICHEM LABORATORIES LTD |
| 23603 | A | II | March 22, 2010 | TORRENT PHARMACEUTICALS LTD |
| Parameters | Details |
|---|---|
| Strength | 10 MG |
| Excipients used | Colloidal silicon dioxide (NF), ethylcellulose (NF), hydrogenated castor oil (NF), hydroxypropyl methylcellulose (USP), magnesium stearate (NF), mannitol (USP), microcrystalline cellulose (NF), povidone (USP), and yellow ferric oxide (NF) |
| Composition of coating material | - |
| Composition of caspule shell | - |
| Pharmaceutical Development | - |
| Manufacture of the product | - |
| Tablet / Capsule Image |

|
| Appearance | Round, three-layer tablet: one white layer between two yellow layers, debossed with X10 |
| Imprint code / Engraving / Debossment | Debossed with X10 |
| Score | No score |
| Color | One white layer between two yellow layers |
| Shape | Round, three-layer tablet |
| Dimension | 8 mm |
| Mfg by | - |
| Mfg for | - |
| Marketed by | Aventis Pharma Limited (EU, UK) |
| Distributed by | Covis Pharmaceuticals, Inc (US) |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021287 | 1 | 6149940 | August 22, 2017 | - | - | - | - | Download |
| N021287 | 1 | 6149940*PED | February 22, 2018 | - | - | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 100 | 0.01 N HCl | 900 | 1, 2, 12, 20 hours | June 18, 2007 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | Xatral XL | Download |
| UK | - | |
| US | APOTEX INC (ANDA # 079013)* | Download |
| US | AUROBINDO PHARMA LTD (ANDA # 079060)* | |
| US | INVAGEN PHARMS (ANDA # 090284)* | |
| US | MYLAN (ANDA # 079014)* | Download |
| US | SUN PHARMA GLOBAL (ANDA # 079057)* | Download |
| US | TEVA PHARMS (ANDA # 079056)* | Download |
| US | TORRENT PHARMS (ANDA # 079054)* | Download |
| US | UNICHEM LABS LTD (ANDA # 203192)* | |
| US | UROXATRAL | Download |
| Xatral 2.5 mg is available in EU. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
