| Active Ingredient | ACETYLCYSTEINE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CETYLEV | NDA#207916 | ARBOR PHARMS LLC | TABLET, EFFERVESCENT;ORAL | 500MG, 2.5GM | 500MG, 2.5GM | January 29, 2016 | - | - | Type 3 - New Dosage Form | STANDARD ; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
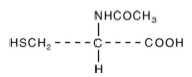 |
| Chemical Name | N-acetyl-L-cysteine |
| CAS No | 616-91-1 |
| Molecular Formula | C5H9NO3S |
| Molecular Weight | 163.2 |
| Appearance | a white crystalline powder |
| Solubility | API is freely soluble in water, alcohol, practically insoluble in chloroform and in ether. |
| Water Solubility | 5.09 mg/mL |
| Polymorphism | - |
| pKa (Strongest Acidic) | 9.52 (at 25 °C) |
| pKa (Strongest Basic) | - |
| Log P | - |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | 109-110°C |
| BCS Class | I |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | CETYLEV is indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in patients with acute ingestion or from repeated supratherapeutic ingestion (RSI). |
| Dosage and Administration | Refer FDA Label |
| Mechanism of action | Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Acetaminophen doses of 150 mg/kg or greater have been associated with hepatotoxicity. Acetylcysteine probably protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite of acetaminophen. |
| Absorption | After administration of a single oral dose of 11 grams of CETYLEV (dissolved in 300 mL of water) to 29 healthy adult subjects, the mean Cmax (CV%) was 26.5 (29) mcg/mL and mean (CV) AUCinf was 186 (29) hr•mcg/mL. The median (range) time to reach Cmax (Tmax) was 2 (1 to 3.5) hours. |
| Food Effect | - |
| Distribution | The steady-state volume of distribution (Vd) following administration of an intravenous dose of acetylcysteine was 0.47 liter/kg. The protein binding for acetylcysteine ranges from 66% to 87 %. |
| Metabolism | Acetylcysteine (i.e., N-acetylcysteine) undergoes extensive first pass metabolism and is postulated to form cysteine and disulfides (N,N-diacetylcysteine and N-acetylcysteine). Cysteine is further metabolized to form glutathione and other metabolites. |
| Elimination |
After a single oral dose of [35S]-acetylcysteine 100 mg, between 13 to 38% of the total radioactivity administered was recovered in urine within 24 hours. In a separate study, renal clearance was estimated to be approximately 30% of total body clearance. In healthy subjects given a single oral dose of 11 grams of CETYLEV, the mean (CV%) terminal plasma half-life (T1/2) was 18.1 (22%) hours. |
| Peak plasma time (Tmax) | 2 hours |
| Half life | 18.1 hours |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 14304 | A | II | July 21, 1999 | MOEHS IBERICA SL |
| 21789 | A | II | July 10, 2008 | NIPPON RIKA CO LTD |
| 5091 | A | II | July 4, 1983 | FIS FABBRICA ITALIANA SINTETICI SPA |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 500 mg | 2.5 gram | |
| Excipients used | sodium bicarbonate, maltodextrin, lemon flavor, sucralose, peppermint flavor, and edetate disodium. | ||
| Composition of coating material | - | ||
| Composition of caspule shell | - | ||
| Pharmaceutical Development |
CETYLEV (acetylcysteine) effervescent tablets for oral solution contain 500 mg or 2.5 grams of acetylcysteine. 500MG tablet contains 320MG of Sodium bicarbonate and 88MG of Sodium. 2.5MG tablet contains 1600MG of Sodium bicarbonate and 438MG of Sodium. |
||
| Manufacture of the product | - | ||
| Tablet / Capsule Image | |||
| Appearance | white, round, flat tablets with a lemon mint flavor, debossed with “I” on one side and plain on other side. Flavour: LEMON (Lemo n Mint) | white, round, flat tablets with a lemon mint flavor, debossed with “O” on one side and plain on other side. Flavour: LEMON (Lemo n Mint) | |
| Imprint code / Engraving / Debossment | “I” on one side and plain on other side. | “O” on one side and plain on other side. | |
| Score | no score | no score | |
| Color | WHITE | WHITE | |
| Shape | ROUND | ROUND | |
| Dimension | 16mm | 25mm | |
| Mfg by | - | ||
| Mfg for | Arbor Pharmaceuticals LLC (US) | ||
| Marketed by | - | ||
| Distributed by | - | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N207916 | 1 | 8747894 | May 8, 2032 | - | DP | U-1373 | - | Download |
| N207917 | 1 | 9427421 | May 8, 2032 | - | DP | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| - | - | Develop a dissolution method | - | - | July 28, 2016 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov, www.drug.com |
