| Active Ingredient | ABEMACICLIB |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VERZENIO | 208716 | ELI LILLY AND CO | TABLET;ORAL | 50 MG, 100 MG, 150 MG, 200 MG | 200 MG | September 28, 2017 | September 28, 2021 | _ | Type 1 - New Molecular Entity | PRIORITY | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
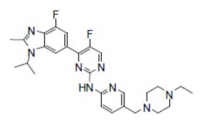 |
| Chemical Name | 2-Pyrimidinamine, N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl] |
| CAS No | 1231929-97-7 |
| Molecular Formula | C27H32F2N8 |
| Molecular Weight | 506.59 |
| Appearance | White to yellow powder |
| Solubility | Sparingly soluble in ethanol. The solubility is pH dependent |
| Water Solubility | 0.0159 mg/mL (Predicted). , Practically insoluble in water |
| Polymorphism | The crystal forms have been thoroughly characterized (physically and chemically) by X-ray powder diffraction, solid-state C NMR spectroscopy, solution-state H NMR spectroscopy, polarized light microscopy, thermal analysis and moisture sorption analysis. The thermodynamically most stable neat polymorph, Form III, was chosen for development. The current manufacturing (crystallization) process delivers Form III in highly crystalline and phase pure form. |
| pKa (Strongest Acidic) | 10.27 (Predicted) |
| pKa (Strongest Basic) | 7.94 (Predicted) |
| Log P | 4.25 (Predicted) |
| Identification | IR/Raman |
| Degradation | Solutions of abemaciclib did not exhibit significant degradation across the pH range indicating that abemaciclib is not susceptible to hydrolysis. Solutions of abemaciclib in oxidative conditions underwent significant degradation, indicating that abemaciclib is susceptible to oxidative conditions in solution. The abemaciclib solutions containing trace metals did not undergo any degradation indicating that abemaciclib is not susceptible to trace metal catalysed degradation. Significant degradation of abemaciclib was observed in all of the light-exposed solutions. The rate of photodegradation was pH dependent with the slowest photodegradation occurring at low pH. |
| Hygroscopic | Non-hygroscopic |
| Photostability study | - |
| Melting Point | - |
| BCS Class | III |
| Manufacture of API | Abemaciclib is synthesized by a convergent multiple step synthesis using commerciallyavailable well defined GMP starting materials with acceptable specifications. Abemaciclib is isolated after controlled crystallization to produce Form III and milled to produce the required particle size distribution. The synthesis and the proposed starting materials are accepted as concluded in Scientific Advice |
| Parameters | Details |
|---|---|
| Indications and Usage | VERZENIO™ is a kinase inhibitor indicated: • in combination with fulvestrant for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy. • as monotherapy for the treatment of adult patients with HRpositive,HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. |
| Dosage and Administration |
VERZENIO tablets are taken orally with or without food. • Recommended starting dose in combination with fulvestrant:150 mg twice daily. • Recommended starting dose as monotherapy: 200 mg twice daily. • Dosing interruption and/or dose reductions may be required based on individual safety and tolerability. |
| Mechanism of action |
Abemaciclib is an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6). These kinases are activated upon binding to D-cyclins. In estrogen receptor-positive (ER+) breast cancer cell lines, cyclin D1 and CDK4/6 promote phosphorylation of the retinoblastoma protein (Rb), cell cycle progression, and cell proliferation. In vitro, continuous exposure to abemaciclib inhibited Rb phosphorylation and blocked progression from G1 into S phase of the cell cycle, resulting in senescence and apoptosis. In breast cancer xenograft models, abemaciclib dosed daily without interruption as a single agent or in combination with antiestrogens resulted in reduction of tumor size. |
| Absorption | The absolute bioavailability of abemaciclib after a single oral dose of 200 mg is 45% (19% CV). The median Tmax of abemaciclib is 8.0 hours (range: 4.1-24.0 hours). |
| Food Effect |
A high-fat, high-calorie meal (approximately 800 to 1000 calories with 150 calories from protein, 250 calories from carbohydrate, and 500 to 600 calories from fat) administered to healthy subjects increased the AUC of abemaciclib plus its active metabolites by 9% and increased Cmax by 26%. |
| Distribution |
In vitro, abemaciclib was bound to human plasma proteins, serum albumin, and alpha-1-acid glycoprotein in a concentration independent manner from 152 ng/mL to 5066 ng/mL. In a clinical study, the mean (standard deviation, SD) bound fraction was 96.3% (1.1) for abemaciclib, 93.4% (1.3) for M2, 96.8% (0.8) for M18, and 97.8% (0.6) for M20. The geometric mean systemic volume of distribution is approximately 690.3 L (49% CV).In patients with advanced cancer, including breast cancer, concentrations of abemaciclib and its active metabolites M2 and M20 in cerebrospinal fluid are comparable to unbound plasma concentrations. |
| Metabolism |
Hepatic metabolism is the main route of clearance for abemaciclib. Abemaciclib is metabolized to several metabolites primarily by cytochrome P450 (CYP) 3A4, with formation of N-desethylabemaciclib (M2) representing the major metabolism pathway. Additional metabolites include hydroxyabemaciclib (M20), hydroxy-N-desethylabemaciclib (M18), and an oxidative metabolite (M1). M2, M18, and M20 are equipotent to abemaciclib and their AUCs accounted for 25%, 13%, and 26% of the total circulating analytes in plasma, respectively. |
| Elimination |
The geometric mean hepatic clearance (CL) of abemaciclib in patients was 26.0 L/h (51% CV), and the mean plasma elimination half-life for abemaciclib in patients was 18.3 hours (72% CV). Excretion After a single 150 mg oral dose of radiolabeled abemaciclib, approximately 81% of the dose was recovered in feces and approximately 3% recovered in urine. The majority of the dose eliminated in feces was metabolites. |
| Peak plasma time (Tmax) | 8.0 hours (range: 4.1-24.0 hours) |
| Half life | 18.3 hours (72% CV) |
| Bioavailability | 45% (19% CV) |
| Age, gender | Based on a population pharmacokinetic analysis in patients with cancer, age (range 24-91 years), gender (134 males and 856 females), and body weight (range 36-175 kg) had no effect on the exposure of abemaciclib. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details | ||||
|---|---|---|---|---|---|
| Strength | 50 MG | 100 MG | 150 MG | 200 MG | |
| Excipients used | Microcrystalline cellulose 102, microcrystalline cellulose 101, lactose monohydrate, croscarmellose sodium, sodium stearyl fumarate, silicon dioxide. | ||||
| Composition of coating material | Polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow, and iron oxide red | ||||
| Composition of caspule shell | - | ||||
| Pharmaceutical Development |
As indicated above, several crystalline forms of abemaciclib free base were observed during a comprehensive polymorph screening. Crystalline Form III is non-hygroscopic and is thermo-dynamically more stable than the other neat crystal forms, solvates and hydrates and was chosen for commercialization. The crystalline Form III of abemaciclib was used in all the clinical studies. Potential for phase transformation of abemaciclibwas evaluated during formulation development and ruled out.Abemaciclib crystalline Form III is highly soluble; the highest dose strength (200 mg) is soluble in less than 250 mL across the pH range 1 to 6.8. In vitro studies indicated that abemaciclib has moderate permeability. Therefore, abemaciclib was considered by the applicant a Biopharmaceutics Classification System (BCS) 3 molecule. In silico modeling suggested that the absorption of abemaciclib is not solubility/dissolution limited and is independent of particle size over a wide range.Nonetheless, an acceptance criterion for particle size was set using the x90 parameter to mitigate any residual risk to absorption. In addition, pharmacokinetic analysis of data from studies showed that the absorption of abemaciclib is not formulation dependent (not solubility/dissolution-limited) and is independent of active substance particle size within the sizes that were used in the clinical studies. |
||||
| Manufacture of the product |
The manufacturing process consists of five main steps: pre-blending, feeding of the raw materials, continuous mixing and direct compression, and film-coating. Core tablets manufactured from this continuous process are then coated in a traditional batch tablet coating process. |
||||
| Tablet / Capsule Image | |||||
| Appearance | Oval beige tablet with “Lilly” debossed on one side and “50” on the other side | Oval white to practically white tablet with “Lilly” debossed on one side and “100” on the other side | Oval yellow tablet with “Lilly” debossed on one side and “150” on the other side | Oval beige tablet with “Lilly” debossed on one side and “200” on the other side | |
| Imprint code / Engraving / Debossment | “Lilly” debossed on one side and “50” on the other side | “Lilly” debossed on one side and “100” on the other side | “Lilly” debossed on one side and “150” on the other side | “Lilly” debossed on one side and “200” on the other side | |
| Score | No score | No score | No score | No score | |
| Color | Beige | White to practically white | Yellow | Beige | |
| Shape | Oval | Oval | Oval | Oval | |
| Dimension | 5.2 × 9.5 (Tablet weight 144.2 mg) | 6.6 × 12.0 (Tablet weight 291.2 mg) | 7.5 × 13.7 (Tablet weight 432.6 mg) | 15 mm | |
| Mfg by | - | ||||
| Mfg for | - | ||||
| Marketed by | Lilly USA | ||||
| Distributed by | - | ||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N208716 | 1 | 7855211 (Submission on 10/16/2017) | December 15, 2029 | DS | DP | U-2132 U-2135 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 0.01 N HCl | 900 | 5, 10, 15, 20 and 30 | November 16, 2017 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | VERZENIO | Download |
| UK | VERZENIO | Download |
| US | VERZENIO | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
