| Active Ingredient | PREGABALIN |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LYRICA | (NDA) 021446 | PF PRISM CV | CAPSULE;ORAL | 25MG , 50 MG, 75 MG, 100 MG, 150 MG, 200 MG, 225 MG, 300 MG | 300 MG | December 30, 2004 | _ | _ | 1 New molecular entity (NME) | P Priority review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
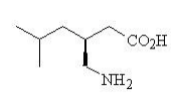 |
| Chemical Name | (S)-3-(aminomethyl)-5-methylhexanoic acid |
| CAS No | 148553-50-8 |
| Molecular Formula | C8H17NO2 |
| Molecular Weight | 159.23 |
| Appearance | White to off-white, crystalline solid |
| Solubility | Freely soluble in both basic and acidic aqueous solutions |
| Water Solubility | Freely soluble in water, 11.3 mg/mL (Predicted) |
| Polymorphism | Pregabalin exists as a single anhydrous and not solvated crystal form. Polymorph screening performed during development did not indicate any possible solid-state form transition events. |
| pKa (Strongest Acidic) | pKa1 of 4.2 and a pKa2 of 10.6 |
| pKa (Strongest Basic) | - |
| Log P | (n-octanol/0.05M phosphate buffer) at pH 7.4 is – 1.35 |
| Identification | IR and chiral HPLC |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | Not light sensitive |
| Melting Point | - |
| BCS Class | I |
| Manufacture of API | Pregabalin is prepared at three different sites through a two-step chemical synthesis. The first step consists of the synthesis of pregabalin racemate from commercially available starting materials. The undesired R enantiomer is subsequently removed by a classical resolution method. Pregabalin crude is terminally purified by recrystallisation from isopropyl alcohol and water to give pregabalin. |
| Parameters | Details |
|---|---|
| Indications and Usage | LYRICA is indicated for: • Neuropathic pain associated with diabetic peripheral neuropathy (DPN) • Postherpetic neuralgia (PHN) • Adjunctive therapy for adult patients with partial onset seizures • Fibromyalgia • Neuropathic pain associated with spinal cord injury |
| Dosage and Administration |
• For all indications, begin dosing at 150 mg/day. • Dosing recommendations: DPN Pain: 3 divided doses per day, Maximum dose: 300 mg/day within 1 week PHN : 2 or 3 divided doses per day, Maximum dose: 300 mg/day within 1 week. Maximum dose of 600 mg/day Adjunctive Therapy for Adult Patients with Partial Onset Seizures:2 or 3 divided doses per day, Maximum dose of 600 mg/day Fibromyalgia: 2 divided doses per day, Maximum dose 300 mg/day within 1 week. Maximum dose of 450 mg/day Neuropathic Pain Associated with Spinal Cord Injury: 2 divided doses per day, Maximum dose 300 mg/day within 1 week. Maximum dose of 600 mg/day • Dose should be adjusted in patients with reduced renal function. • Oral Solution Concentration and Dispensing |
| Mechanism of action | LYRICA (pregabalin) binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin's anti-nociceptive and antiseizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium-dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium channel trafficking and/or reducing calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord. While pregabalin is a structural derivative of the inhibitory neurotransmitter gammaaminobutyric acid (GABA), it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not augment GABAA responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake. |
| Absorption | Following oral administration of LYRICA capsules under fasting conditions, peak plasma concentrations occur within 1.5 hours. Pregabalin oral bioavailability is greater than or equal to 90% and is independent of dose. Following single- (25 to 300 mg) and multiple- dose (75 to 900 mg/day) administration, maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) values increase linearly. Following repeated administration, steady state is achieved within 24 to 48 hours. Multiple-dose pharmacokinetics can be predicted from single-dose data. |
| Food Effect | The rate of pregabalin absorption is decreased when given with food, resulting in a decrease in Cmax of approximately 25% to 30% and an increase in Tmax to approximately 3 hours. However, administration of pregabalin with food has no clinically relevant effect on the total absorption of pregabalin. Therefore, pregabalin can be taken with or without food. |
| Distribution | Pregabalin does not bind to plasma proteins. The apparent volume of distribution of pregabalin following oral administration is approximately 0.5 L/kg. Pregabalin is a substrate for system L transporter which is responsible for the transport of large amino acids across the blood brain barrier. Although there are no data in humans, pregabalin has been shown to cross the blood brain barrier in mice, rats, and monkeys. In addition, pregabalin has been shown to cross the placenta in rats and is present in the milk of lactating rats. |
| Metabolism | Pregabalin undergoes negligible metabolism in humans. Following a dose of radiolabeled pregabalin, approximately 90% of the administered dose was recovered in the urine as unchanged pregabalin. The N-methylated derivative of pregabalin, the major metabolite of pregabalin found in urine, accounted for 0.9% of the dose. In preclinical studies, pregabalin (Senantiomer) did not undergo racemization to the R-enantiomer in mice, rats, rabbits, or monkeys. |
| Elimination | Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug with a mean elimination half-life of 6.3 hours in subjects with normal renal function. Mean renal clearance was estimated to be 67.0 to 80.9 mL/min in young healthy subjects. Because pregabalin is not bound to plasma proteins this clearance rate indicates that renal tubular reabsorption is involved. Pregabalin elimination is nearly proportional to creatinine clearance (CLcr) |
| Peak plasma time (Tmax) | 1.5 hours |
| Half life | 6.3 hours |
| Bioavailability | 90% |
| Age, gender | Population pharmacokinetic analyses of the clinical studies showed that the relationship between daily dose and LYRICA drug exposure is similar between genders. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 22111 | A | II | October 23, 2008 | MSN PHARMACHEM PRIVATE LTD |
| 22128 | A | II | November 27, 2008 | SUN PHARMACEUTICAL INDUSTRIES LTD |
| 22165 | A | II | November 10, 2008 | MYLAN LABORATORIES LTD |
| 22223 | A | II | November 24, 2008 | CHANGZHOU PHARMACEUTICAL FACTORY |
| 22242 | A | II | December 1, 2008 | TEVA PHARMACEUTICAL INDUSTRIES LTD |
| 22330 | A | II | December 17, 2008 | LUPIN LTD |
| 22485 | A | II | February 3, 2009 | MYLAN LABORATORIES LTD |
| 22925 | A | II | July 8, 2009 | CADILA HEALTHCARE LTD |
| 23342 | I | II | November 30, 2009 | ZHEJIANG JIUZHOU PHARMACEUTICAL CO LTD |
| 24196 | A | II | September 21, 2010 | LABORATORIO CHIMICO INTERNAZIONALE SPA |
| 24292 | A | II | September 30, 2010 | ALEMBIC PHARMACEUTICALS LTD |
| 24700 | A | II | March 3, 2011 | SRINI PHARMACEUTICALS LTD |
| 25064 | A | II | June 21, 2011 | MSN PHARMACHEM PRIVATE LTD |
| 25083 | A | II | June 18, 2011 | MYLAN LABORATORIES LT |
| 26062 | A | II | May 2, 2012 | KOPRAN LTD |
| 26246 | A | II | July 31, 2012 | CTX LIFE SCIENCES PVT LTD |
| 26317 | A | II | August 27, 2012 | MACLEODS PHARMACEUTICALS LTD |
| 26387 | A | II | September 14, 2012 | AUROBINDO PHARMA LTD |
| 26673 | A | II | January 23, 2013 | LUPIN LTD |
| 27354 | A | II | July 29, 2013 | HETERO DRUGS LTD |
| 27792 | A | II | December 26, 2013 | CADILA HEALTHCARE LTD |
| 27839 | A | II | January 6, 2014 | INTAS PHARMACEUTICALS LTD |
| 27955 | A | II | February 12, 2014 | ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD |
| 28123 | A | II | March 18, 2014 | SHASUN PHARMACEUTICALS LTD |
| 28306 | A | II | May 16, 2014 | DIVIS LABORATORIES LTD |
| 28396 | A | II | June 30, 2014 | PIRAMAL ENTERPRISES LTD |
| 28663 | A | II | September 19, 2014 | HIKAL LTD |
| 29168 | A | II | March 11, 2015 | ZHEJIANG APELOA JIAYUAN PHARMACEUTICAL CO LTD |
| 29688 | A | II | August 12, 2015 | HIKAL LTD |
| Parameters | Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strength | 150 MG | 25 MG | 50 MG | 300 MG | 75 MG | 100 MG | 200 MG | 225 MG | |
| Excipients used | lactose monohydrate (16.50 mg ), cornstarch (16.75 mg), and talc (16.77 mg) | lactose monohydrate (35 mg), cornstarch (20 mg), and talc (20 mg) | lactose monohydrate (70 mg), cornstarch (40 mg), and talc (40 mg) | lactose monohydrate (33 mg), cornstarch (33.5 mg), and talc (33.5 mg) | lactose monohydrate (8.25 mg), cornstarch (8.375 mg), and talc (8.375 mg) | lactose monohydrate (11 mg), cornstarch, and talc | lactose monohydrate (22 mg), cornstarch, and talc | lactose monohydrate (24.75 mg), cornstarch, and talc | |
| Composition of coating material | - | ||||||||
| Composition of caspule shell | Gelatin and titanium dioxide, white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | Gelatin and titanium dioxide, orange capsule shells contain red iron oxide, white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | Gelatin and titanium dioxide, white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | Gelatin and titanium dioxide, orange capsule shells contain red iron oxide, white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | Gelatin and titanium dioxide, orange capsule shells contain red iron oxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | Gelatin and titanium dioxide, orange capsule shells contain red iron oxide, white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide, imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide | |||
| Pharmaceutical Development | Pregabalin being a highly soluble and highly permeable compound, the oral dosage form developed is of standard formulation. The excipients selected are of PhEur quality, commonly used for this type of dosage form and were selected based on compatibility studies with the drug substance. Particle size distribution of the drug substance has been shown not to adversely impact drug product manufacture and performance. Particle size is part of the specification, but is notexpected to be a critical parameter with regards to the bioavailability of the capsules, taking into account the water solubility of pregabalin. This has been confirmed by the broad range particle size distribution of drug substance lots used in clinical studies | ||||||||
| Manufacture of the product | The method of manufacture involves the following operations: delumping of pregabalin and of the excipients, blending and encapsulation. In order toaccommodate the range of dosage strengths, 2 powder blends formulation are usedfor commercial manufacturing. Formulation series A containing 25% w/w pregabalin is used to produce 25 and 50 mg capsules and formulation series C containing 75% w/w pregabalin is used to produce the other strengths. | ||||||||
| Tablet / Capsule Image |
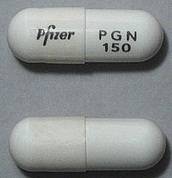
|
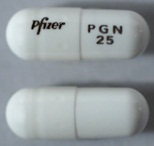
|
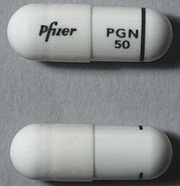
|
|
|

|
|
|
|
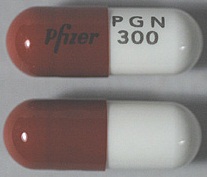
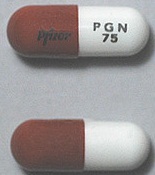
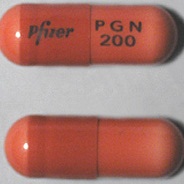
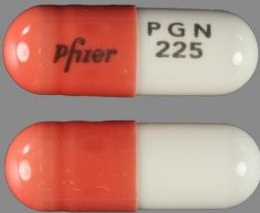
| Appearance | White hard gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 150" on the body | White, hard-gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 25" on the body | White, hard-gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 50" and an ink band on the body | White/orange hard gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 300" on the body | White/orange hard gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 75" on the body | Orange, hard-gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 100" on the body | Light orange hard gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 200" on the body | White/light orange hard gelatin capsule printed with black ink "Pfizer" on the cap, "PGN 225" on the body | |
| Imprint code / Engraving / Debossment | Printed with black ink "Pfizer" on the cap, "PGN 150" on the body | Printed with black ink "Pfizer" on the cap, "PGN 25" on the body | Printed with black ink "Pfizer" on the cap, "PGN 50" and an ink band on the body | Printed with black ink "Pfizer" on the cap, "PGN 300" on the body | Printed with black ink "Pfizer" on the cap, "PGN 75" on the body | Printed with black ink "Pfizer" on the cap, "PGN 100" on the body | Printed with black ink "Pfizer" on the cap, "PGN 200" on the body | Printed with black ink "Pfizer" on the cap, "PGN 225" on the body | |
| Score | No score | No score | No score | No score | No score | No score | No score | No score | |
| Color | White | White | White | White/light orange | White/orange | Orange | Light orange | White/light orange | |
| Shape | Capsule | Capsule | Capsule | Capsule | Capsule | Capsule | Capsule | Capsule | |
| Dimension | 18 mm | - | - | - | - | - | - | - | |
| Mfg by |
Pfizer Pharmaceuticals LLC (US) Pfizer Manufacturing Deutschland GmbH (EU) |
||||||||
| Mfg for | - | ||||||||
| Marketed by | Pfizer Limited (EU) | ||||||||
| Distributed by | Pfizer (US) | ||||||||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N021446 | 1 | 6001876 | December 30, 2018 | - | - | U - 55 | Y | Download |
| N021446 | 1 | 6001876 | December 30, 2018 | - | - | U - 55 | Y | Download |
| N021446 | 1 | 6197819 | December 30, 2018 | Y | Y | - | - | Download |
| N021446 | 1 | RE41920 | December 30, 2018 | - | - | U - 1250 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) with sinker | 50 | 0.06 N HCl | 900 | 10, 20, 30 and 45 | January 15, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | LYRICA | Download |
| UK | LYRICA | Download |
| US | ACTAVIS ELIZABETH (ANDA # 091025)* (Tentative Approval) | |
| US | ALEMBIC PHARMS LTD (ANDA # 203459)*(Tentative Approval) | |
| US | APOTEX INC (ANDA # 203022)*(Tentative Approval) | |
| US | LUPIN LTD (ANDA # 091040)* (Tentative Approval) | |
| US | LYRICA | Download |
| US | MACLEODS PHARMS LTD (ANDA # 205924)*(Tentative Approval) | |
| US | MYLAN PHARMS INC (ANDA # 091228)* (Tentative Approval) | |
| US | SANDOZ INC (ANDA # 091229)*(Tentative Approval) | |
| US | TEVA PHARMS (ANDA # 091219)* (Tentative Approval) | |
| US | TEVA PHARMS (ANDA # 091224)* (Tentative Approval) | |
| US | WATSON LABS INC (ANDA # 091221)* (Tentative Approval) | |
| US | WOCKHARDT USA (ANDA # 091222)* (Tentative Approval) |
| Date of first authorisation in EU on: 06 July 2004 |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
